Source-Weill-Cornell-Medicine
New Insights into a Rare Bone Tumor
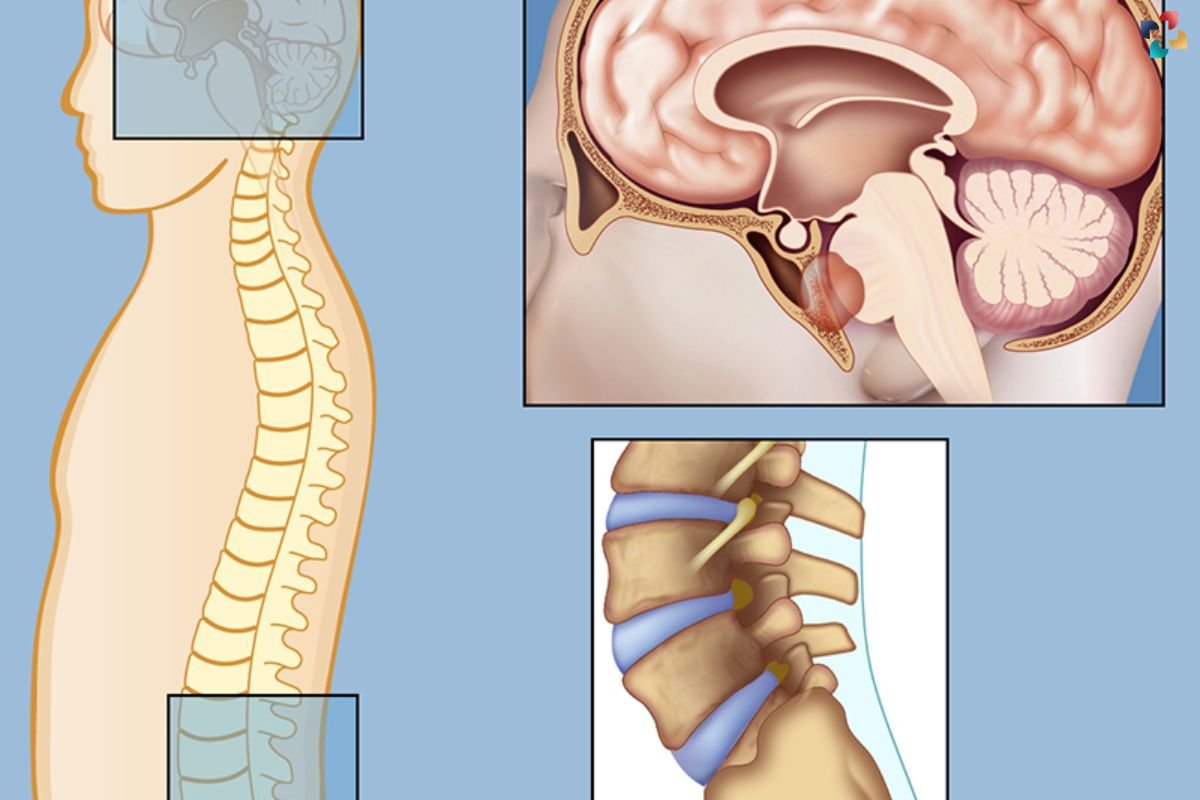
Researchers at Children’s Hospital Los Angeles (CHLA) have made significant strides in understanding the genetic causes of pediatric chordoma, a rare and aggressive bone tumor. Chordomas, which occur in approximately one in a million people annually in the U.S., present unique challenges in children, as they primarily occur at the base of the skull. This location often makes complete surgical removal difficult, necessitating high doses of radiation that can harm the developing brain. A team led by Dr. Xiaowu Gai and Dr. Jaclyn Biegel at CHLA’s Center for Personalized Medicine has published a study revealing two classes of genetic causes for pediatric chordoma, paving the way for better treatment strategies.
Genetic Discoveries and Methodologies
The CHLA team conducted a comprehensive genomic analysis, examining both nuclear DNA and mitochondrial DNA (mtDNA) in tumor samples from 23 pediatric patients. Previous studies primarily focused on adult chordomas, which differ in presentation and behavior from pediatric cases. The researchers identified that poorly differentiated pediatric chordoma is driven by the loss of the SMARCB1 gene, a key component of the SWI/SNF chromatin remodeling complex. This complex plays a crucial role in DNA packaging within cells.
In addition to nuclear DNA, the team explored mtDNA, an area often neglected in past studies. By sequencing the exons of all nuclear genes and the entire mitochondrial genome of the chordoma samples, they discovered significant mtDNA mutations, particularly in NADH (Mitochondrial Complex 1 genes). These findings suggest a potential link between chromatin remodeling and mitochondrial metabolism in chordoma development.
Implications and Future Directions
The study’s results have far-reaching implications for the treatment of chordoma. The identification of short inframe insertions and deletions (indels) in the ARID1B gene, present in 22% of pediatric chordoma patients, highlights a genetic similarity with adult chordoma patients, where 5% exhibited comparable mutations. Both ARID1B and SMARCB1 are part of the SWI/SNF complex, indicating a common pathway that may influence gene expression through chromatin remodeling defects.
Dr. Jaclyn Biegel, a senior author of the study, emphasized the significance of these findings in understanding chordoma’s genetic underpinnings. The study also pointed out the enrichment of mtDNA mutations in pediatric samples, with similar patterns observed in adult cases. Dr. Xiaowu Gai, co-senior author, noted the potential interplay between chromatin remodeling and mitochondrial metabolism in chordoma genesis. This understanding could be crucial in developing targeted therapies for both pediatric and adult patients.
By unraveling these genetic factors, the research offers a crucial first step towards more effective and personalized treatment strategies for chordoma. The study underscores the importance of a dual-genome approach, examining both nuclear and mitochondrial DNA, to uncover the complex mechanisms driving this rare and challenging disease.
Also Read: UCLA Research Unveils Insights Into Autism’s Genetic Basis and Brain Changes