Uric acid, a compound that often garners attention in medical discussions, is a naturally occurring substance in the human body. While it plays a role in essential biological processes, its notoriety primarily stems from its association with gout—a form of inflammatory arthritis. However, the implications of uric acid extend far beyond gout, encompassing various metabolic and cardiovascular diseases. This article explores the biochemistry of uric acid, its physiological roles, the pathological consequences of its dysregulation, and contemporary therapeutic strategies.
The Biochemistry of Uric Acid
1. Formation and Metabolism
It is a heterocyclic compound formed through the degradation of purines, which are vital components of nucleotides in DNA and RNA. The metabolic pathway leading to uric acid involves several enzymatic steps:
- Purine Nucleotide Breakdown: Purine nucleotides (adenine and guanine) are broken down into their respective nucleosides (adenosine and guanosine) and further into purine bases (hypoxanthine and xanthine).
- Xanthine Oxidase Activity: The enzyme xanthine oxidase catalyzes the oxidation of hypoxanthine to xanthine and subsequently xanthine to uric acid.
It is the end product of purine metabolism in humans and some other animals. Unlike many other mammals, humans lack the enzyme uricase, which further degrades uric acid to the more soluble compound allantoin. This evolutionary trait means that humans must excrete this acid through the kidneys.
2. Excretion
Approximately two-thirds of this acid produced daily is excreted by the kidneys, while the remaining one-third is eliminated via the gastrointestinal tract. The balance between production and excretion is crucial for maintaining normal serum uric acid levels, typically ranging from 3.5 to 7.2 mg/dL for men and 2.6 to 6.0 mg/dL for women.
Physiological Roles of Uric Acid

Despite its often negative reputation, it has several physiological roles:
1. Antioxidant Properties
It is a potent antioxidant, accounting for nearly half of the antioxidant capacity of human plasma. It scavenges reactive oxygen species (ROS) and protects against oxidative stress, which can damage cells and tissues. This antioxidant function is particularly important in the brain, where uric acid may help protect neurons from oxidative damage.
2. Immune System Modulation
It can influence the immune response. Crystals of monosodium urate (MSU), formed when it concentrations are excessively high, can activate the innate immune system. These crystals are recognized by pattern recognition receptors (PRRs) on immune cells, leading to the production of pro-inflammatory cytokines. This mechanism is central to the pathogenesis of gout but also illustrates uric acid’s broader role in immune regulation.
Pathophysiology of Hyperuricemia and Gout
1. Hyperuricemia
Hyperuricemia, defined as elevated serum uric acid levels above 7 mg/dL, is the primary risk factor for gout. The condition can result from increased uric acid’s production, decreased excretion, or a combination of both. Factors contributing to hyperuricemia include:
- Genetic Predisposition: Genetic mutations can affect its metabolism and excretion.
- : High purine intake from red meat, seafood, and alcohol can elevate these acid levels.
- Obesity and Metabolic Syndrome: Insulin resistance and obesity are strongly associated with reduced renal excretion of acid.
- Renal Insufficiency: Impaired kidney function can hinder uric acid clearance.
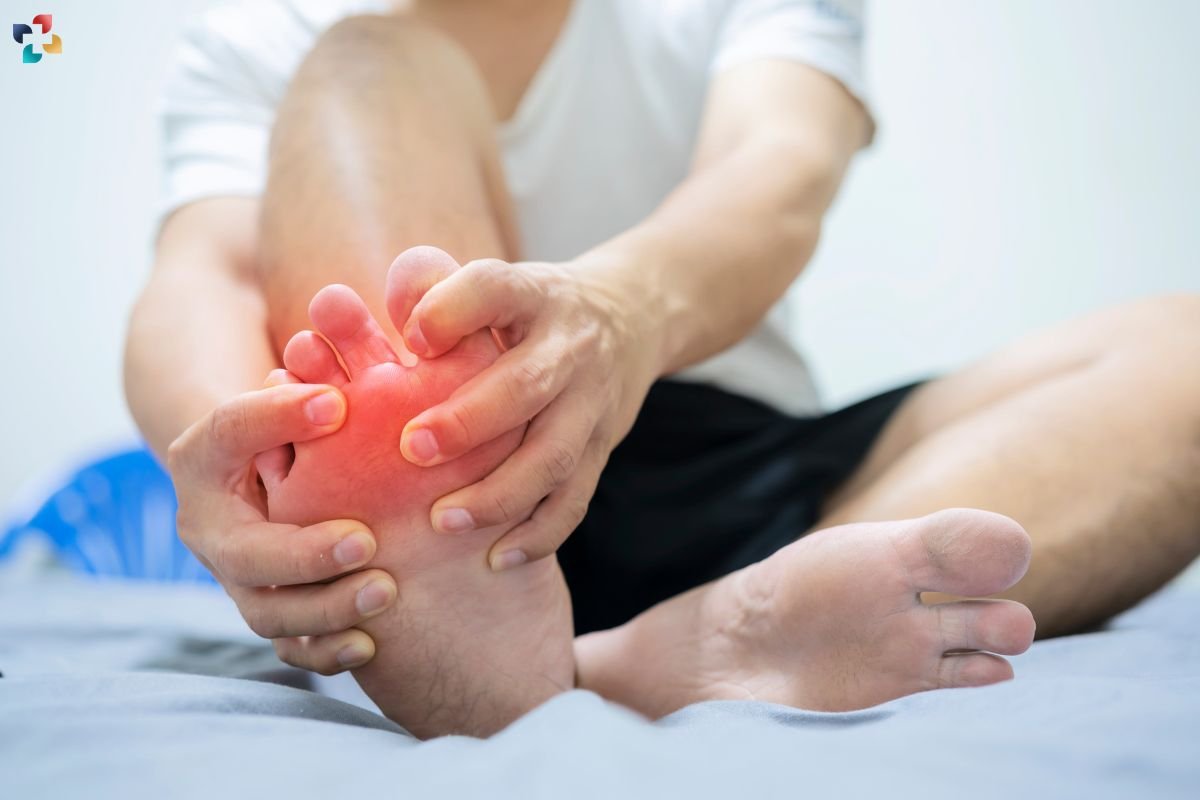
2. Gout
Gout is characterized by acute episodes of joint inflammation caused by the deposition of MSU crystals in synovial fluid and tissues. These episodes, known as gout flares, are extremely painful and typically affect the big toe but can involve other joints.
Pathogenesis of Gout
- Crystal Formation: Persistent hyperuricemia leads to the supersaturation of uric acid in body fluids, promoting the formation of MSU crystals.
- Crystal Deposition: MSU crystals deposit in joints, triggering an inflammatory response. The innate immune system detects these crystals, leading to the activation of NLRP3 inflammasomes.
- Inflammatory Response: Activated inflammasomes initiate the production of interleukin-1β (IL-1β) and other pro-inflammatory cytokines, recruiting neutrophils to the site and causing intense inflammation and pain.
Chronic Gout and Tophaceous Gout
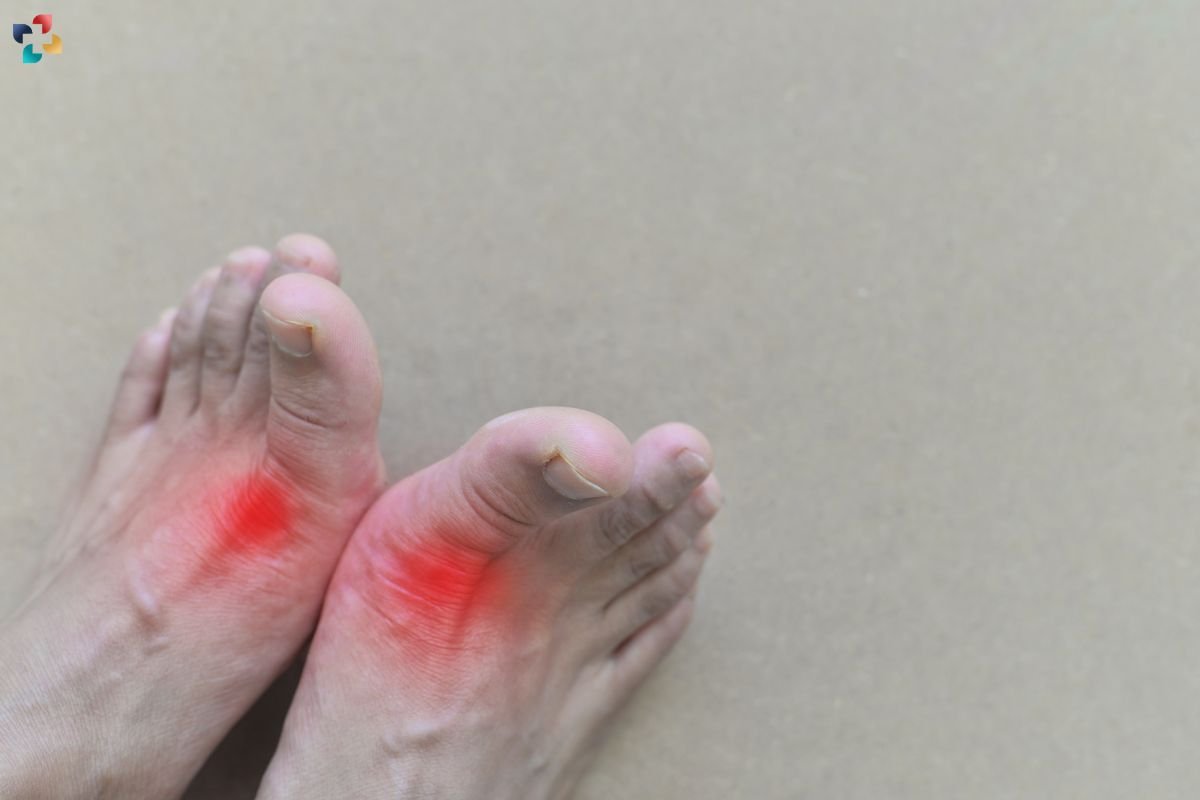
Repeated gout flares can result in chronic gout, characterized by persistent low-grade inflammation and the formation of tophi—large aggregates of MSU crystals in soft tissues and joints. Tophi can cause joint deformity and damage, significantly impairing mobility and quality of life.
Beyond Gout: Uric Acid and Metabolic Diseases
Emerging research suggests that this acid is not only a marker of gout but also a contributor to various metabolic and cardiovascular diseases.
1. Metabolic Syndrome
Metabolic syndrome, a cluster of conditions including obesity, insulin resistance, hypertension, and dyslipidemia, is often associated with hyperuricemia. Elevated uric acid levels may exacerbate insulin resistance and promote adipogenesis, contributing to the development and progression of metabolic syndrome.
2. Cardiovascular Disease
Hyperuricemia is an independent risk factor for hypertension and cardiovascular disease. Elevated uric acid levels can lead to endothelial dysfunction, increased oxidative stress, and inflammation—all of which are critical factors in the pathogenesis of atherosclerosis and hypertension.
3. Chronic Kidney Disease
Uric acid can contribute to the progression of chronic kidney disease (CKD). Hyperuricemia can induce renal vasoconstriction, reduce renal blood flow, and promote renal inflammation and fibrosis, accelerating the decline in kidney function.
Therapeutic Approaches
1. Lifestyle Modifications
Dietary and lifestyle changes are fundamental in managing hyperuricemia and preventing gout flares. Recommendations include:
- Diet: Reducing intake of purine-rich foods (e.g., red meat, seafood), fructose, and alcohol.
- Hydration: Increasing fluid intake to enhance uric acid excretion.
- Weight Management: Achieving and maintaining a healthy weight to reduce insulin resistance and uric acid levels.
2. Pharmacological Treatments
Several medications are used to manage hyperuricemia and gout:
- Xanthine Oxidase Inhibitors: Allopurinol and febuxostat inhibit xanthine oxidase, reducing this acid production.
- Uricosurics: Probenecid and lesinurad enhance renal excretion of uric acid.
- Uricase: Pegloticase, a recombinant uricase, converts uric acid to allantoin, used in severe refractory gout.
3. Anti-Inflammatory Drugs

During acute gout flares, anti-inflammatory medications are essential to control pain and inflammation:
- Nonsteroidal Anti-Inflammatory Drugs (NSAIDs): Commonly used for rapid relief.
- Corticosteroids: Effective in reducing inflammation in patients who cannot take NSAIDs.
- Colchicine: An anti-inflammatory agent specifically used for gout.
4. Novel Therapies
Research into novel therapies targeting uric acid metabolism and inflammation is ongoing. Potential approaches include:
- IL-1 Inhibitors: Drugs like anakinra and canakinumab target IL-1β, reducing inflammation associated with MSU crystals.
- Renal Transport Modulators: New agents targeting renal urate transporters may improve uric acid excretion.
Conclusion
Uric acid, once primarily associated with gout, is now recognized for its broader implications in human health. While it serves beneficial roles as an antioxidant and immune modulator, its dysregulation can lead to hyperuricemia and a cascade of pathological conditions, including gout, metabolic syndrome, cardiovascular disease, and chronic kidney disease. Understanding the complexities of uric acid metabolism and its impact on health is essential for developing effective prevention and treatment strategies. Ongoing research continues to unveil new therapeutic targets, promising improved management and outcomes for individuals affected by hyperuricemia and its associated diseases.