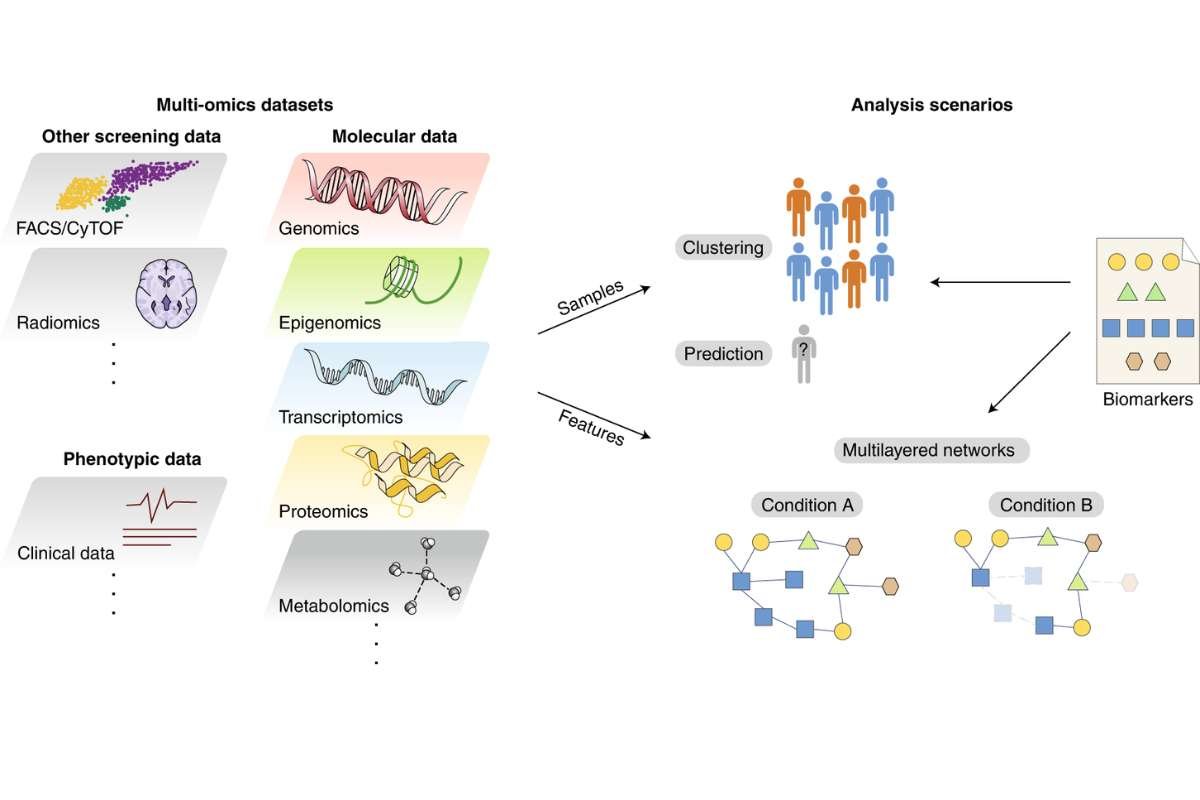
A 2026 Nature Metabolism multi-omics study by Morigny and colleagues shows that cancer cachexia arises from coordinated, time‑linked molecular changes across muscle, fat, and liver. The research identifies early signals and potential treatment targets through comprehensive multi‑omics analysis.
Multi-Omics Trace Cachexia Across Tissues
Cachexia, a life-threatening wasting syndrome affecting many cancer patients, emerges as a coordinated, bodywide process rather than isolated tissue failure, according to research published this month in Nature Metabolism. The study integrates transcriptomic, proteomic and metabolomic data to map how tumors trigger systemic catabolism over time.
Led by bioengineer Pierre Morigny, the team analyzed multiple organs in mouse models bearing cachexia-inducing tumors. The results show early metabolic disruptions in one tissue can precede and amplify damage elsewhere, accelerating muscle loss and energy imbalance.
“Cachexia is not a single-organ disease,” the authors wrote, describing it as a synchronized response driven by tumor-host signaling. The work reframes a long-standing challenge in oncology by revealing how tissues communicate during disease progression.
Early Signals Predict Disease Progression
The multi-omics study used longitudinal sampling to identify molecular patterns that distinguish early, pre‑cachectic states from advanced disease. In skeletal muscle, early mitochondrial dysfunction and oxidative stress emerged before widespread protein breakdown and atrophy.
Adipose tissue followed a different but complementary path, showing increased lipolysis and inflammation that worsened as tumors progressed. Metabolomic data revealed shifts in lipid and energy intermediates consistent with systemic fuel depletion.
“These signatures offer a window for early intervention,” Morigny said in a statement accompanying the study, noting that molecular changes were detectable before severe weight loss occurred. The integrated dataset may support biomarker development to identify patients at risk.
The analysis relied on high-throughput RNA sequencing and mass spectrometry, combined through bioinformatics pipelines to track spatial and temporal changes. The approach allowed the team to link tissue-specific changes to overall disease severity.
Pathways Point to New Treatment Targets
The multi-omics study highlights regulatory pathways that may be vulnerable to therapy. It shows that signaling systems involved in inflammation and protein degradation, including NF‑kB and the ubiquitin‑proteasome pathway, were activated in parallel across muscle and fat.
“That coordination suggests we need multi-target strategies,” said Dr. Laura Chen, an oncologist at Memorial Sloan Kettering Cancer Center who was not involved in the research. Treating muscle alone may not be enough if metabolic signals from other tissues persist, she said.
The authors report that tumor-derived factors differed between cancer models, influencing how cachexia unfolded. This finding supports personalized approaches based on tumor biology and patient metabolism, rather than one-size-fits-all treatments.
While the work is preclinical, experts say the multi-omics study sets a new benchmark for understanding complex cancer syndromes. “This is a systems‑level map we have not had before,” Chen said. “It provides a framework to test therapies earlier and with greater precision.”
The researchers caution that clinical translation will require validation in human patients. Still, they argue that a multi-omics study could become a standard approach for investigating cachexia and other metabolic disorders marked by organ crosstalk.
Cachexia contributes to reduced treatment tolerance and higher mortality in cancer, making early detection critical. By defining molecular warning signs, the study offers hope for interventions that preserve strength and quality of life.