Source-American-Institute-for-Cancer-Research
Epigenetic changes are garnering increasing attention in the field of genetics and medicine for their profound influence on gene expression and cellular function. Unlike alterations in the DNA sequence itself, epigenetic changes involve modifications to the structure of DNA and its associated proteins, impacting how genes are activated or silenced without altering the underlying genetic code. This article delves into the intricacies of epigenetic changes, exploring their mechanisms, impact on health and disease, and potential implications for personalized medicine.
Deciphering the Mechanisms of Epigenetic Changes
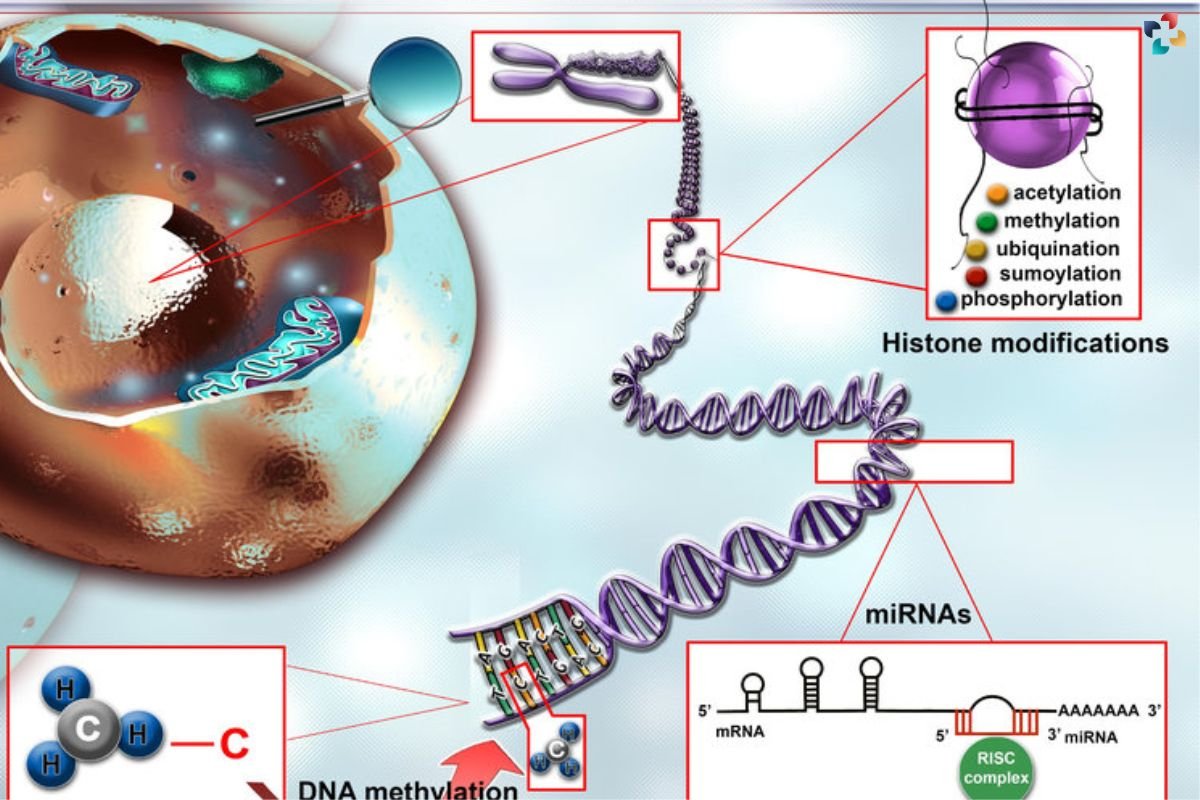
Epigenetic changes encompass a diverse array of modifications, including DNA methylation, histone modifications, and non-coding RNA-mediated regulation. DNA methylation involves the addition of methyl groups to specific regions of the DNA molecule, typically cytosine bases within CpG dinucleotides, leading to gene silencing. Histone modifications, on the other hand, alter the packaging of DNA around histone proteins, influencing chromatin structure and accessibility to transcriptional machinery. Non-coding RNAs, such as microRNAs and long non-coding RNAs, play crucial roles in post-transcriptional gene regulation, modulating mRNA stability and translation.
Epigenetic changes, as intricate as they are pivotal, constitute a multifaceted regulatory system governing gene expression and cellular identity. Among the diverse repertoire of epigenetic modifications, DNA methylation stands out as a fundamental mechanism for gene regulation. The addition of methyl groups to specific regions of the DNA molecule, particularly cytosine bases within CpG dinucleotides, orchestrates gene silencing by impeding the binding of transcriptional machinery and recruiting proteins involved in chromatin condensation. This dynamic process of DNA methylation is tightly regulated by DNA methyltransferases (DNMTs), enzymes responsible for adding methyl groups, and demethylases, which facilitate their removal, thereby maintaining genomic integrity and cellular homeostasis.
In parallel, histone modifications exert profound effects on chromatin structure and function, shaping the accessibility of DNA to transcriptional regulators. Histones, the proteins around which DNA is wound, undergo various post-translational modifications, including acetylation, methylation, phosphorylation, and ubiquitination. These modifications alter the electrostatic interactions between histones and DNA, loosening or compacting chromatin structure and modulating the recruitment of transcriptional activators or repressors. The dynamic interplay between histone-modifying enzymes, such as histone acetyltransferases (HATs) and histone deacetylases (HDACs), finely tunes gene expression programs in response to environmental cues and cellular signaling pathways.
Non-coding RNAs add another layer of complexity to epigenetic regulation, serving as versatile mediators of gene expression control. MicroRNAs (miRNAs) and long non-coding RNAs (lncRNAs) participate in post-transcriptional gene regulation by binding to target mRNAs, thereby modulating their stability and translation efficiency. By fine-tuning gene expression networks, non-coding RNAs play critical roles in diverse biological processes, including development, differentiation, and disease pathogenesis. Dysregulation of non-coding RNA-mediated regulation has been implicated in various diseases, highlighting their potential as diagnostic biomarkers and therapeutic targets.
In summary, the mechanisms of epigenetic changes are marvels of biological regulation, orchestrating the intricate dance of gene expression and cellular identity. By deciphering the complexities of DNA methylation, histone modifications, and non-coding RNA-mediated regulation, researchers are gaining insights into the molecular underpinnings of health and disease, paving the way for innovative diagnostic and therapeutic strategies in the era of personalized medicine.
Unraveling the Impact of Epigenetic Changes on Health
Epigenetic changes play a pivotal role in various physiological processes, including embryonic development, cellular differentiation, and tissue-specific gene expression. They help orchestrate the intricate interplay between genetic predispositions and environmental influences, shaping an individual’s phenotype and susceptibility to disease. Dysregulation of epigenetic mechanisms has been implicated in the pathogenesis of numerous health conditions, including cancer, cardiovascular disease, neurodegenerative disorders, and autoimmune diseases. For example, aberrant DNA methylation patterns are frequently observed in cancer cells, contributing to oncogene activation and tumor suppressor gene silencing.
Exploring the Dynamic Nature of Epigenetic Changes
One of the most fascinating aspects of epigenetic changes is their dynamic nature, capable of being modulated by various environmental factors and lifestyle choices. Environmental exposures, such as diet, stress, pollutants, and toxins, can induce alterations in epigenetic marks, influencing gene expression patterns and contributing to disease susceptibility. Conversely, behavioral interventions, such as exercise, meditation, and dietary modifications, have been shown to exert beneficial effects on epigenetic regulation, promoting health and well-being. Understanding the dynamic interplay between epigenetics and the environment holds promise for developing personalized strategies for disease prevention and treatment.
Implications for Disease Diagnosis and Treatment
The burgeoning field of epigenetics has significant implications for disease diagnosis, prognosis, and therapeutic interventions. Epigenetic biomarkers hold promise for early detection and risk stratification of various diseases, providing valuable insights into disease progression and response to treatment. Moreover, epigenetic-targeted therapies, such as DNA methyltransferase inhibitors and histone deacetylase inhibitors, are being explored as novel treatment modalities for cancer and other diseases characterized by epigenetic dysregulation. By targeting specific epigenetic modifications, these therapies aim to restore normal gene expression patterns and halt disease progression, offering new avenues for precision medicine.
Conclusion: Embracing the Complexity of Epigenetic Changes
In conclusion, epigenetic changes represent a fascinating and complex layer of gene regulation with profound implications for human health and disease. By deciphering the mechanisms underlying epigenetic regulation and understanding their dynamic nature, researchers are unraveling the intricate interplay between genetics, environment, and disease susceptibility. The ongoing exploration of epigenetics holds promise for revolutionizing disease diagnosis, prognosis, and treatment, ushering in a new era of personalized medicine aimed at optimizing health outcomes for individuals across the lifespan.
FAQs
1. What are epigenetic changes?
Epigenetic changes refer to modifications to the structure of DNA and its associated proteins that influence gene expression without altering the underlying genetic code. These changes play a critical role in regulating various biological processes and can be influenced by environmental factors and lifestyle choices.
2. How do epigenetic changes impact gene expression?
Epigenetic changes, such as DNA methylation and histone modifications, can alter the accessibility of DNA to transcriptional machinery, leading to either gene activation or silencing. Non-coding RNAs also play a role in post-transcriptional gene regulation, modulating mRNA stability and translation efficiency.
3. What factors can influence epigenetic changes?
Epigenetic changes can be influenced by a variety of factors, including environmental exposures (such as diet, stress, and toxins), lifestyle choices (such as exercise and smoking), age, and genetic predisposition. These factors can interact with one another to modulate epigenetic regulation and impact health outcomes.
4. Are epigenetic changes reversible?
Yes, many epigenetic changes are reversible and can be dynamically modulated in response to environmental cues and cellular signaling pathways. Lifestyle interventions, such as diet, exercise, and stress management, have been shown to impact epigenetic regulation and promote health and well-being.
5. How are epigenetic changes relevant to disease?
Dysregulation of epigenetic mechanisms has been implicated in the pathogenesis of various diseases, including cancer, cardiovascular disease, neurodegenerative disorders, and autoimmune diseases. Understanding the role of epigenetic changes in disease susceptibility and progression may lead to the development of novel diagnostic biomarkers and therapeutic interventions.