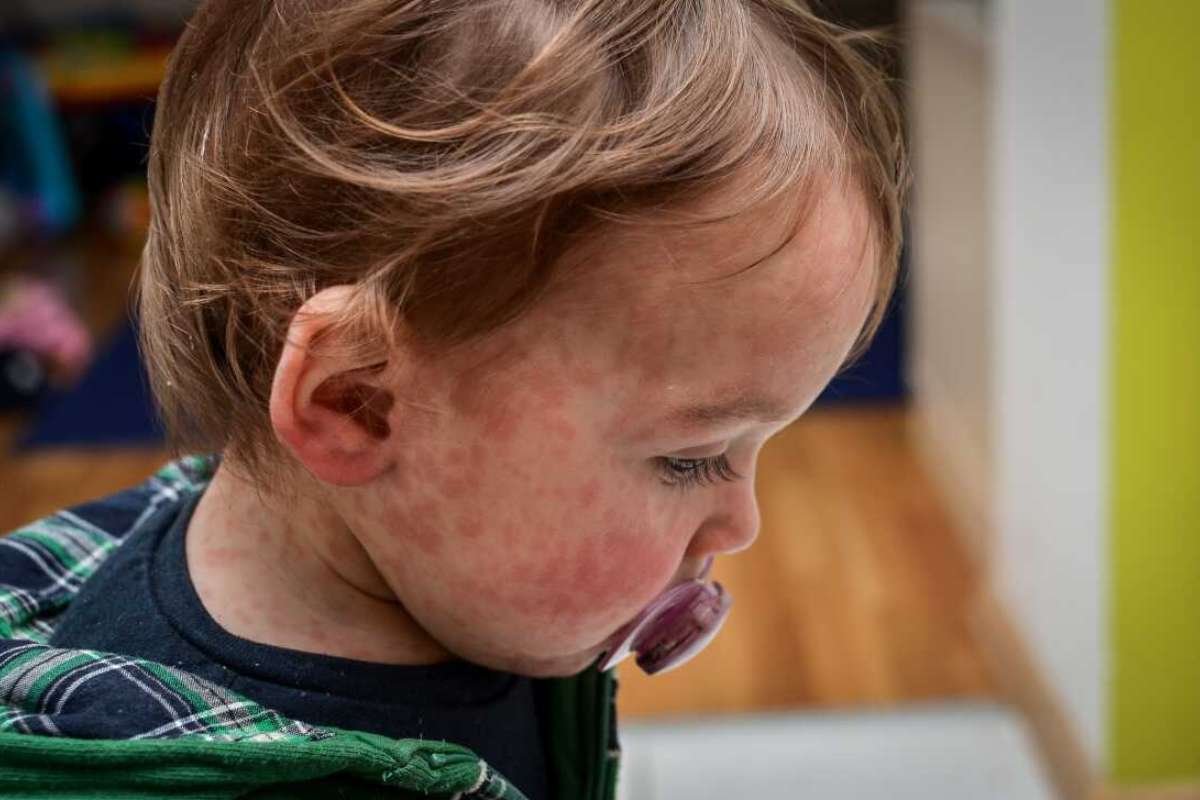
For the first time, a respiratory syncytial virus (RSV) vaccination appears ready to aid thousands of people. It’s been decades in the making, but on May 3 the US Food and Drug Administration (FDA) approved the vaccine for use in adults over the age of 60.
Availability of the Vaccine
The vaccine would be accessible at US pharmacies and clinics by the fall if it were to be approved by the US Centres for Disease Control and Prevention, which might happen by June. Respiratory Syncytial Virus often doesn’t cause much more harm than a cold, but it can be fatal for elderly people, especially those with underlying medical issues.
RSV kills over 14,000 seniors over the age of 65 annually in the US alone, and tens of thousands more are hospitalized. If the virus spreads, young children are also at risk. In the US, severe infections are thought to be the cause of 100 to 500 baby fatalities annually; however, the number is significantly greater in underdeveloped nations.
USFDA approves the world’s first vaccine for highly contagious Respiratory Syncytial Virus
Why RSV Vaccine?
It is a significant health issue, which is why efforts to develop a Respiratory Syncytial Virus vaccine have lasted for decades without bearing fruit. The pharmaceutical company GSK’s Arexvy vaccine offers a defense against RSV-induced lower respiratory tract illness (LRTD).
Tony Wood, the Chief Scientific Officer at GSK in the UK, declares that “today marks a turning point in our effort to reduce the significant burden of RSV.” “Our current priorities are to advance regulatory review in other nations while ensuring that eligible older adults in the US can access the vaccine as soon as possible.”
The ability to produce antibodies from a more in-depth analysis of the protein that RSV employs to merge with and infect cells propelled the development of the vaccine. In recent years, COVID vaccines have also been developed using a similar methodology. After a nearly 25,000-person experiment in which half of the subjects received Arexvy and the other half received placebos, the vaccine was approved.