1. New Insights Into Astrocyte Response to Spinal Cord Injuries and Strokes
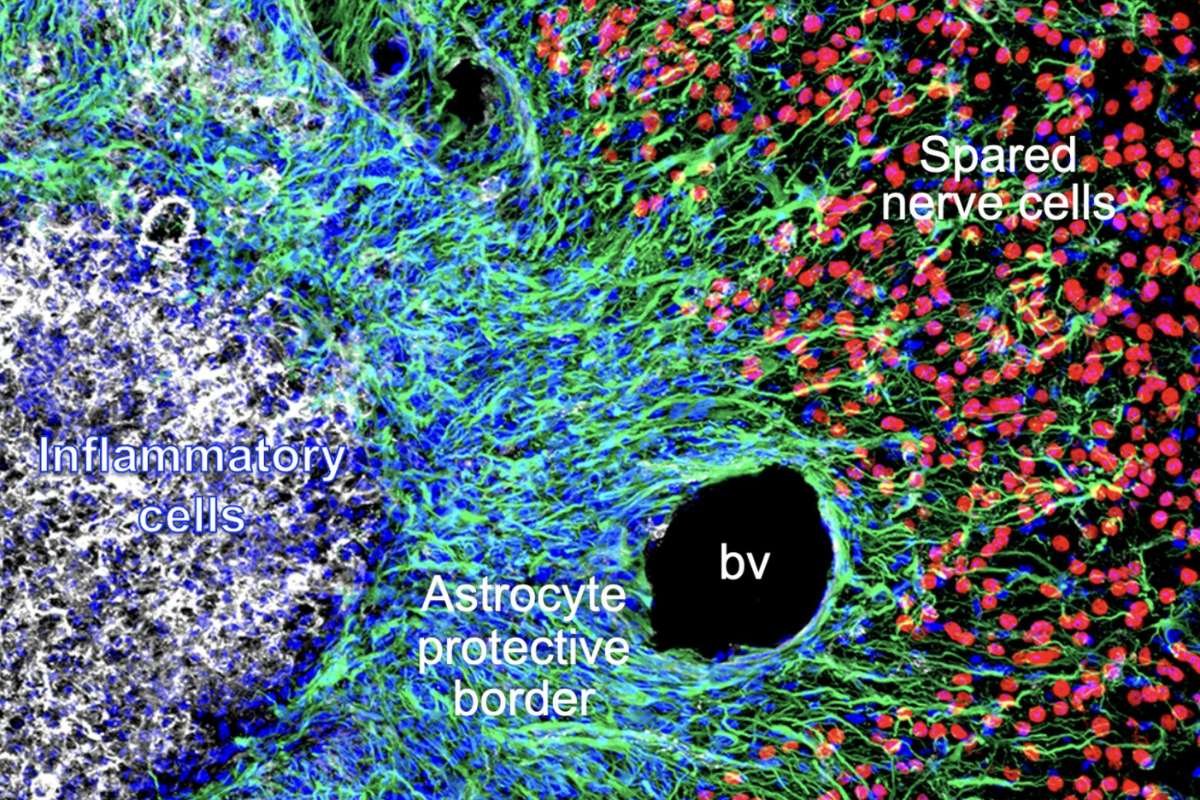
Recent research has shed light on the crucial role astrocytes play in responding to central nervous system (CNS) damage, particularly following spinal cord injuries or strokes. Astrocytes, star-shaped glial cells known for their support functions in the brain, have long been observed to proliferate around lesions caused by such injuries. These newly formed astrocytes create protective borders around damaged tissue, but their precise contributions to wound repair have remained unclear.
Previous studies highlighted the importance of these cells in clearing neurotransmitters and supporting the formation of synapses in healthy brains, but their role in injury recovery is gaining renewed interest. A better understanding of how astrocytes respond to tissue damage could open doors to more effective therapies for stroke and spinal cord injury patients.
Research at UCLA Sheds New Light on Astrocyte Functions
In a recent study published in Nature Neuroscience, researchers from the University of California, Los Angeles (UCLA) examined the response of astrocytes to CNS injuries in adult mice. Led by Michael Sofroniew and his team, the study found that 90% of the astrocytes forming borders around lesions originated from proliferating local astrocytes, while 10% came from oligodendrocyte progenitor cells. This discovery challenges previous assumptions that astrocytes hinder recovery, instead revealing their vital role in protecting nerve cells.
Sofroniew and his colleagues initially embarked on this research to investigate whether astrocytes prevent or support nerve cell regrowth. Their earlier findings, published in 2016 and 2018, indicated that astrocytes play a supportive role in nerve cell regeneration and tissue repair. The current study builds on that work, offering deeper insights into how astrocytes respond to damage and form neuroprotective borders.
Astrocytes as Key Players in Neuroprotection
The study reveals that these astrocytic borders serve as barriers to protect healthy neurons from inflammatory cells that infiltrate damaged areas. By surrounding these inflammatory cells, astrocytes clean up cellular debris and reduce the risk of infection. These cells, now referred to as “wound repair astrocytes,” help prevent further damage to neighboring nerve cells.
Using a combination of histological tissue sections and gene expression analysis, the researchers explored how astrocytes in the brain and spinal cord of adult mice respond to injuries. Their findings suggest that mature astrocytes de-differentiate, proliferate, and genetically reprogram themselves to support tissue repair. While astrocytes were once viewed as detrimental after traumatic injuries or strokes, this study emphasizes their neuroprotective role. The researchers also noted that astrocytic functions may become less effective with age or in certain disease conditions, highlighting the importance of further research into their role in recovery.
In conclusion, the study’s findings offer valuable insights into astrocytes’ potential in therapeutic interventions for CNS injuries, paving the way for new strategies to improve recovery following strokes and spinal cord damage.
2. UroGen Pharma Initiates Phase 3 Trial for UGN-103 in Treating Non-Muscle Invasive Bladder Cancer
UroGen Pharma Ltd., a biotechnology company focused on developing innovative cancer treatments, has announced the commencement of its Phase 3 clinical trial for UGN-103, a next-generation mitomycin-based drug. This investigational treatment, specifically designed for low-grade intermediate-risk non-muscle invasive bladder cancer (LG-IR-NMIBC), marks a significant advancement in bladder cancer therapeutics. The first patient has now been dosed in the UTOPIA study, which will evaluate the drug’s safety and efficacy.
Liz Barrett, UroGen’s President and CEO, expressed optimism about the drug’s potential, stating, “UGN-103 offers several improvements in manufacturing, convenience, and cost, providing a novel treatment option for patients. This milestone reflects our commitment to advancing care for those with LG-IR-NMIBC.” UGN-103 utilizes UroGen’s proprietary RTGel® technology, a sustained-release hydrogel that holds promise for enhancing the therapeutic profile of existing cancer drugs by allowing longer exposure of the bladder tissue to the medication.
UGN-103: A Game-Changing Approach to Bladder Cancer Treatment
The U.S. Food and Drug Administration (FDA) approved the Investigational New Drug (IND) application for UGN-103 in April 2024, enabling clinical trials in adults with LG-IR-NMIBC. The UGN-103 trial follows the upcoming potential FDA approval of UGN-102, another drug developed by UroGen using its RTGel technology, with a decision anticipated in early 2025. UroGen’s ongoing clinical research into these non-surgical treatment options aims to reduce the need for invasive procedures, such as transurethral resection of bladder tumor (TURBT), the current standard of care.
The UTOPIA study is a single-arm, multicenter trial designed to enroll 87 patients with LG-IR-NMIBC. The study will assess the effectiveness of UGN-103 based on the complete response rate at the three-month mark, with participants receiving six weeks of weekly treatments. Patients who show no detectable disease after three months will enter a follow-up period to monitor their progress for up to 15 months. The study’s primary objective is to determine the drug’s ability to prevent disease recurrence and progression.
Potential Impact on Non-Muscle Invasive Bladder Cancer Care
Bladder cancer is a prevalent and recurring condition, particularly among older individuals. In the U.S., it is the second most common urologic cancer in men, with around 22,000 new cases of LG-IR-NMIBC diagnosed each year. Many of these patients experience recurrence, requiring repeated surgeries and ongoing treatments. UroGen’s UGN-103 and UGN-102 treatments offer a promising alternative to surgery, leveraging the company’s sustained-release technology to deliver effective local therapy.
In addition to its potential therapeutic benefits, UGN-103 may also streamline the drug manufacturing process, simplifying procedures and reducing costs. UroGen has already received a Notice of Allowance from the United States Patent and Trademark Office for UGN-103, with a patent expected to be granted by December 2041. This intellectual property protection will give UroGen the ability to continue innovating in the bladder cancer treatment space.
As UroGen Pharma progresses in its clinical trials, its pioneering RTGel technology has the potential to transform the treatment landscape for bladder cancer patients, offering hope for more convenient and effective non-surgical options.
3. Myrobalan Therapeutics Secures $24 Million to Develop Groundbreaking CNS Therapies
Myrobalan Therapeutics, a biotechnology company focused on developing innovative neurorestorative therapies, has successfully raised $24 million in Series A financing. The funding round was spearheaded by Co-win Ventures, with participation from both new and existing investors, including Guan Zi Equity Investment, 3E Bioventures Capital, and AB Magnitude Ventures Group. The funds will be directed toward advancing Myrobalan’s efforts to develop oral therapies that can repair and restore neural function in patients with degenerative central nervous system (CNS) diseases, such as multiple sclerosis (MS), Alzheimer’s disease (AD), and amyotrophic lateral sclerosis (ALS).
Historically, CNS drug development has faced significant challenges, particularly in crossing the blood-brain barrier and creating treatments with long-term efficacy. Myrobalan aims to overcome these obstacles by developing highly selective, orally available compounds that target demyelination and neuroinflammation, two major contributors to CNS conditions. With these new therapies, Myrobalan seeks to provide hope for currently untreatable conditions that affect millions worldwide.
Innovative Approach to CNS Therapy
Myrobalan is developing a range of neurorestorative therapies, focusing on three major targets. One of these is an antagonist to G-protein coupled receptor 17 (GPR17), which promotes remyelination. Another is an inhibitor of colony-stimulating factor-1 receptor (CSF1R), which addresses both demyelination and neuroinflammation. The third is an allosteric tyrosine kinase 2 (TYK2) inhibitor, designed to reduce neuroinflammation. By targeting these mechanisms, the company aims to create treatments that address the underlying causes of various neurological conditions.
Leadership and Vision for the Future
Myrobalan’s leadership team brings together extensive expertise in both research and business development. The company was co-founded by Dr. Wang in 2021, alongside renowned scientists Dr. Zhigang He from Harvard Medical School and Dr. Guoping Feng from MIT’s McGovern Institute for Brain Research. This strong scientific foundation is complemented by Dr. Wang’s experience in leading drug development programs from discovery to commercialization at companies like Curis and GSK. Additionally, Myrobalan has enlisted top-tier business and scientific advisors to guide the company’s strategic development.
Co-founder Dr. He expressed excitement over Myrobalan’s progress, noting that the company’s approach to targeting key drivers of CNS diseases, such as GPR17 and TYK2, is both novel and timely. Investors, including Dr. Xin Huang from Co-win Ventures and Dr. Frank Yan from 3E Bioventures Capital, also voiced their confidence in the company’s potential to revolutionize CNS treatment through its innovative research and drug design.
With its sights set on transforming the future of CNS therapy, Myrobalan Therapeutics is poised to make significant strides in developing first-in-class neurorestorative treatments that could change the lives of patients suffering from degenerative brain conditions.
4. UF Biomedical Engineer Christine Schmidt was inducted into the National Academy of Engineering

University of Florida (UF) distinguished professor Christine Schmidt has been inducted into the 2024 class of the National Academy of Engineering (NAE), one of the highest honors in the field. The induction ceremony took place during the NAE’s annual meeting in Washington, D.C., where Schmidt was recognized for her groundbreaking contributions to neural tissue engineering and wound healing. Her work over the past 25 years has made a significant impact on patients, particularly those suffering from nerve damage caused by injuries.
Schmidt is the Pruitt Family Endowed Chair in UF’s J. Crayton Pruitt Family Department of Biomedical Engineering. Her research focuses on developing innovative materials and therapeutic systems for wound healing and the regeneration of peripheral and spinal nerves. Schmidt’s work has led to the development of two widely used products: the Avance Nerve Repair graft, produced by Axogen, and the VersaWrap graft, which was developed by her startup company, Alafair Biosciences. These products have helped tens of thousands of patients, revolutionizing nerve repair treatments.
A Trailblazer in Biomedical Engineering
Schmidt’s election to the NAE highlights her extraordinary contributions to the field of biomedical engineering. The NAE, which welcomed 114 newly elected members and 21 international members this year, recognizes engineers who have made pioneering advancements in research and industry. Schmidt’s nerve regeneration and wound healing innovations have already benefited injured soldiers, car accident survivors, and cancer patients, making her work highly influential in both medical and scientific communities.
“Dr. Schmidt has forged a path toward nerve regeneration and wound healing that is already having a remarkable impact,” said Forrest Masters, Ph.D., interim dean of UF’s Herbert Wertheim College of Engineering. “We are proud to be her colleagues and congratulate her on this tremendous accomplishment.” Masters emphasized how Schmidt’s work has redefined nerve repair, providing new hope for patients in need of advanced medical solutions.
Leadership and Legacy at the University of Florida
Schmidt joined UF in 2013 after serving at the University of Texas at Austin. During her time at UF, she led the J. Crayton Pruitt Family Department of Biomedical Engineering for over a decade, significantly advancing interdisciplinary research and promoting the application of engineering solutions to biomedical challenges. Under her leadership, the department expanded its national visibility and impact, leading to more opportunities for translating scientific discoveries into real-world medical applications.
Reflecting on her NAE induction, Schmidt expressed gratitude to her colleagues, collaborators, and students, attributing the honor to the support and hard work of her team. “This recognition reflects the dedication and hard work of my incredible team, collaborators, and students, as well as the supportive environment at the University of Florida,” she said. Schmidt added that she looks forward to continuing her work in biomedical engineering, aiming to push the boundaries of science and innovation to further improve patient outcomes.
With her election to the National Academy of Engineering, Schmidt solidifies her role as a leader in biomedical innovation, committed to advancing science and fostering the next generation of bioengineers.