Key Points:
- MRAP2 controls appetite via MC4R.
- Target for obesity treatment.
- International research breakthrough.
Berlin, Oct. 6—Researchers at Leipzig University and Charité – Universitätsmedizin Berlin have identified a key mechanism showing how the MRAP2 protein regulates appetite and body weight. The discovery explains how a protein called MRAP2 (melanocortin 2 receptor accessory protein 2) influences another brain receptor, MC4R (melanocortin-4 receptor), which plays a central role in controlling appetite and energy balance. The findings were published in Nature Communications.
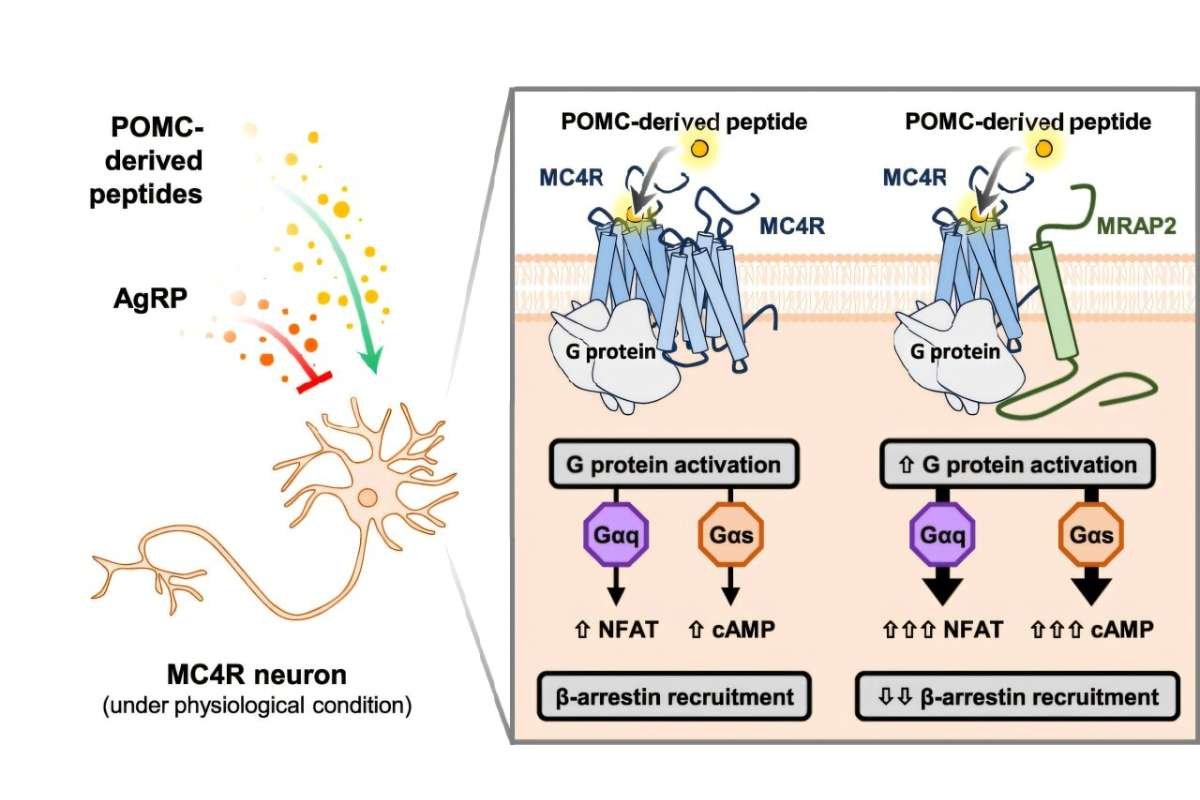
The study was conducted under the Collaborative Research Centre (CRC) 1423 – Structural Dynamics of GPCR Activation and Signaling. Scientists found that MRAP2 directly affects how MC4R functions and is transported within cells, providing new insights into how the body regulates food intake. This discovery explains in detail how the MRAP2 protein regulates appetite.
MC4R is a receptor activated by the hormone MSH, which signals the brain to reduce hunger. Mutations in MC4R are among the most common genetic causes of severe obesity. Researchers say understanding how this receptor works could lead to better treatments for metabolic disorders.
“The knowledge of the 3D structures of the active receptor in interaction with ligands and drugs such as setmelanotide, which we deciphered in an earlier study, has enabled us to better understand the new functional data,” said Dr. Patrick Scheerer, project leader at CRC 1423 and co-author of the study from Charité’s Institute of Medical Physics and Biophysics.
Setmelanotide, an approved drug, activates MC4R and reduces feelings of hunger. “We are proud that CRC 1423 has now also contributed to understanding receptor transport and availability,” said Professor Annette Beck-Sickinger, spokesperson for CRC 1423 and co-author from Leipzig University. Five projects within the centre collaborated on this interdisciplinary research.
Protein found essential for receptor activity
Using modern fluorescence microscopy and single-cell imaging, the team showed that MRAP2 is essential for transporting MC4R to the cell surface, where it can effectively transmit appetite-suppressing signals. Without MRAP2, MC4R remains trapped inside the cell, reducing its ability to communicate with hormones that regulate hunger.
Fluorescent biosensors and confocal imaging revealed how the MRAP2 protein regulates appetite by altering MC4R’s localization and behaviour, uncovering a new level of control over appetite-related signaling. These insights open the door to therapeutic strategies that could mimic or enhance MRAP2’s function, offering potential treatments for obesity and related conditions.
Collaboration drives scientific insight
“This interdisciplinary and international collaboration enabled researchers, using different approaches and experimental methods, to uncover important physiological and pathophysiological aspects of appetite regulation with therapeutic relevance,” said Professor Heike Biebermann, project leader at CRC 1423 and co-lead author from Charité’s Institute of Experimental Pediatric Endocrinology.
Co-lead author Dr. Paolo Annibale, a lecturer at the University of St Andrews in the United Kingdom, said the project allowed scientists to apply advanced microscopy and bioimaging techniques in a physiologically relevant context. “In recent years, we have refined this approach to study molecular processes in cells,” he said.
The research brought together experts in live-cell fluorescence microscopy, molecular pharmacology, and structural biology from Germany, Canada, and the UK. According to the authors, the collaboration highlights how combining multiple scientific disciplines can reveal new principles of receptor regulation and advance the understanding of metabolic health, showing again how the MRAP2 protein regulates appetite.
Visit The Lifesciences Magazine For The Most Recent Information.