Key Points:
- China conducted the first pig lung xenotransplant in a brain-dead human.
- The lung showed initial success but later failed due to immune rejection.
- Lung transplants across species remain difficult and need further research.
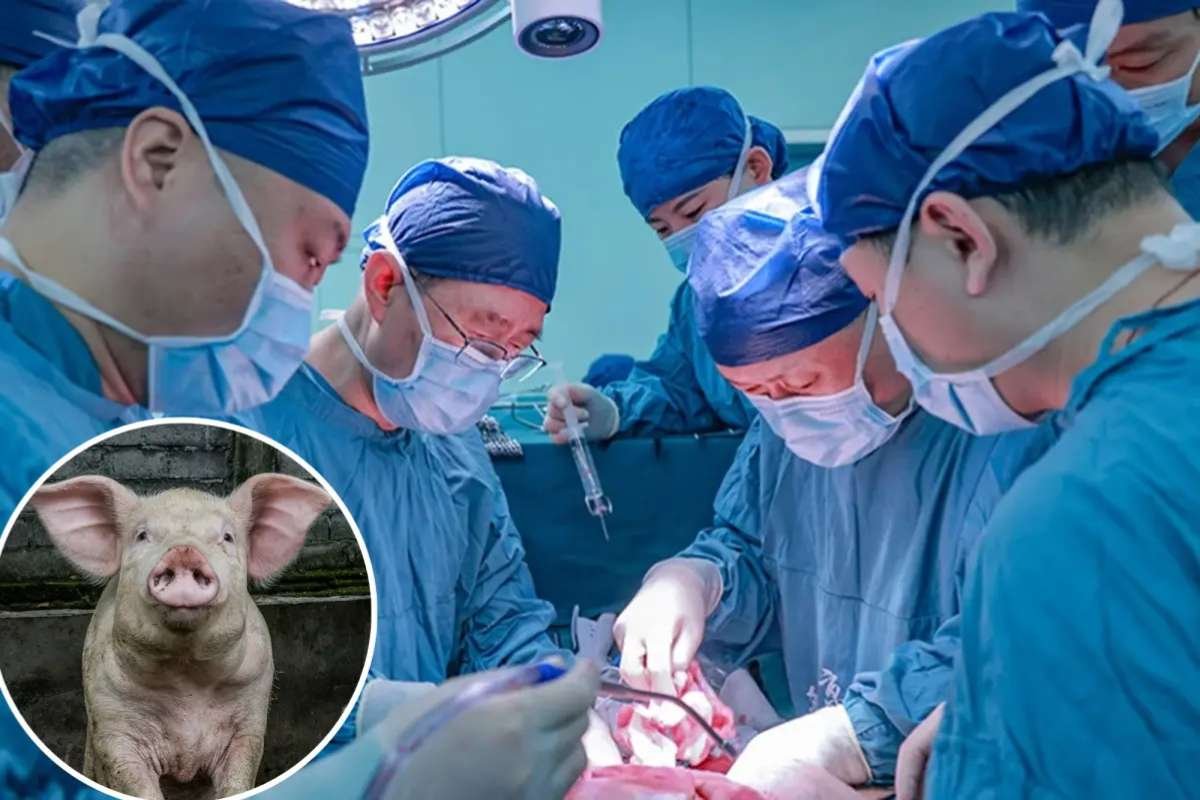
In a first-of-its-kind experiment, Chinese researchers successfully transplanted a gene-edited pig lung into a 39-year-old brain-dead patient, marking a significant milestone in xenotransplantation—the use of animal organs for human transplantation. The procedure, carried out at the First Affiliated Hospital of Guangzhou Medical University, kept the lung in place for nine days before the experiment was ended at the request of the recipient’s family.
The results, published Monday in Nature Medicine, highlight both the promise and the challenges of making pig-to-human lung transplants viable. While the lung initially avoided immediate rejection, it later succumbed to immune responses that damaged the tissue.
Early Signs of Progress, but Major Hurdles Remain
Xenotransplantation has been gaining momentum in recent years, with successful attempts to transplant pig hearts, kidneys, and livers into human recipients. This study, however, marks the first documented case of a Gene-Edited Pig Lung transplant.
The clinical need is urgent: in 2024, 8,236 lung transplants were performed worldwide, yet tens of thousands of patients remain on waitlists. Gene editing has been explored as a way to reduce organ rejection, using CRISPR to eliminate immune-triggering sugars in pig cells while adding human proteins to regulate inflammation.
In the Guangzhou case, the transplanted lung avoided hyperacute rejection — a dangerous immune response that can occur within minutes to hours. However, by the second day, signs of inflammation and antibody activity emerged. Scans later revealed fluid accumulation, a signal of tissue damage, and by day nine, antibody-mediated rejection had firmly set in.
“This is not ready for patients yet,” cautioned Shaf Keshavjee, director of the Toronto Lung Transplant Program, who was not involved in the study. “They’ve shown us important progress, but also how far we have to go.”
Why Lungs Pose Unique Challenges
Compared to hearts or kidneys, lungs are particularly difficult to transplant across species. Because lungs are directly exposed to outside air, they must constantly defend against bacteria, viruses, and environmental irritants. This heightened immune vigilance makes them more prone to rejection, while their fragile structure makes recovery from injury especially difficult.
Surgeons did not remove the patient’s original right lung, leaving it in place as a safeguard. This decision, while ensuring safety, limited the ability to assess whether the pig’s lung could independently sustain life. “Their conclusion that they had a functioning Gene-Edited Pig Lung is a little optimistic,” said Richard Pierson, professor of surgery at Harvard Medical School.
The transplanted lung came from a Bama miniature pig engineered by Clonorgan Biotechnology in Chengdu. It carried six gene edits, including deletions of Gene-Edited Pig Lung cell-surface sugars and additions of human proteins to regulate immune responses. U.S. companies such as eGenesis and Revivicor are pursuing even more extensive modifications, including removing pig viral DNA to lower cross-species infection risks.
Next Steps for Xenotransplantation Research
The Guangzhou team administered an aggressive regimen of more than six immunosuppressive drugs to prevent rejection, a strategy unlikely to be safe for living patients. “The level of immunosuppression was unreal,” said Keshavjee. “In a conscious patient, that would lead to life-threatening infections within days.”
Despite these limitations, experts consider the experiment a valuable step forward. Jiang Shi, one of the study’s authors, emphasized that the research is intended to create a scientific roadmap, not immediate clinical readiness. “Our aim is to build a rigorous pathway toward a safe, durable lung xenograft,” Shi said.
Future experiments will likely test bilateral transplants in brain-dead patients, explore additional gene edits, and refine drug regimens. Scientists are also investigating antibody-neutralizing therapies to buy time for the body to adjust to foreign organs.
For now, the field remains in its early stages. Still, the experiment demonstrates the potential of xenotransplantation to one day expand the donor pool and save lives. “We’re learning that brain-dead patients are a very useful tool for figuring out when genetically modified organs are truly ready for trials,” Keshavjee noted. “This is an important early step, not the finish line.”
Visit The Lifesciences Magazine for the most recent information.