The drug development process is a journey of scientific innovation and rigorous testing designed to bring effective and safe treatments to patients. From initial discovery to post-market safety monitoring, each stage is properly crafted to address safety and quality. This process ensures that new medicines are not only potent against diseases but also safe for widespread use.
In recent years, technological advancements, such as artificial intelligence (AI) and genomic tools, have improved this process and allowed for faster and more precise development of new drugs.
This blog will help you understand the critical stages of the drug development process, tell you the key aspects, and introduce new trends reshaping the pharmaceutical landscape.
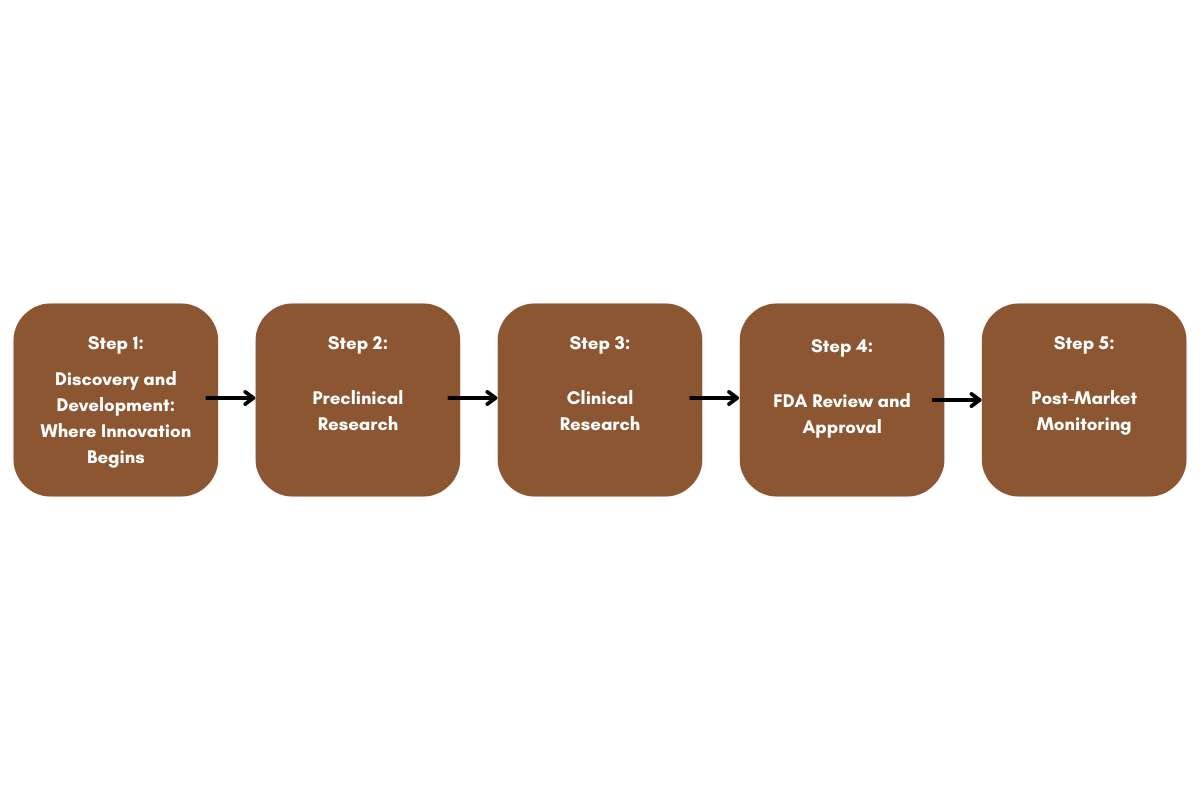
Step 1: Discovery and Development: Where Innovation Begins
The drug development process begins with the discovery phase, where scientists identify therapeutic agents. This stage aims to find compounds that can treat or manage a particular disease.

Discovery
Discovery is a creative and exploratory stage involving:
- Disease Understanding: Advances in understanding disease mechanisms allow researchers to design drugs targeting specific pathways. For instance, studying genetic mutations in cancer helps to lead to targeted therapies.
- Molecular Testing: Thousands of molecular compounds undergo testing to identify promising candidates. New computational models and AI algorithms now accelerate this process, identifying potential leads faster than ever before.
- Repurposing Existing Drugs: Some existing drugs show benefits for conditions other than their original target, which saves years of research and testing.
- Emerging Technologies: Breakthroughs such as CRISPR gene editing and nanotechnology provide new avenues for developing innovative therapies.
Out of thousands of compounds tested, only a few demonstrate the potential for further investigation.
Development
Once a promising compound is identified, the focus shifts to understanding how it works and its safety profile. Researchers conduct detailed studies to examine:
- Absorption, Distribution, Metabolism, and Excretion (ADME): How the body processes the drug.
- Optimal Dosage: Determining the most effective dose with minimal side effects.
- Mechanisms of Action: How the drug interacts with biological systems.
- Toxicity Studies: Identifying any adverse effects and their severity.
Step 2: Preclinical Research

Before testing on humans, preclinical studies are conducted to ensure a drug’s safety. These studies are performed in two main ways:
- In Vitro Studies: Laboratory tests conducted in controlled environments outside living organisms.
- In Vivo Studies: Tests conducted on animal models to understand the drug’s effects on a living system.
Good Laboratory Practices (GLP)
The FDA mandates strict to follow GLP standards for all preclinical research. This includes:
- Using robust protocols and quality assurance systems.
- Maintaining detailed study reports and ensuring regulatory compliance.
Preclinical studies also provide critical data for designing human trials, including dosing strategies and safety precautions.
Step 3: Clinical Research

Clinical trials are important in the drug development process. These human studies are carefully designed to answer questions of safety, efficiency, and using it properly.
Phases of Clinical Trials
- Phase 1: Involves 20-100 healthy volunteers or individuals with the target disease. This phase focuses on safety and determining dosage.
- Phase 2: Up to several hundred participants test the drug’s efficiency and monitor side effects.
- Phase 3: Involves thousands of patients to identify rare adverse events.
- Phase 4: Post-approval studies to gather more data on long-term safety and effectiveness.
FDA Oversight
Before trials commence, developers must file an Investigational New Drug (IND) application with the FDA. The FDA review team, comprising experts from various scientific fields, evaluates the study’s design to ensure participant safety.
Step 4: FDA Review and Approval

If clinical trials are successful, developers submit a New Drug Application (NDA) to the FDA. This document includes data from preclinical and clinical studies, manufacturing details, proposed labeling, and safety updates.
Thorough Review Process
The FDA review team evaluates the application over 6-10 months, examining:
- Clinical data for safety and efficacy.
- Manufacturing processes to ensure product quality.
- Compliance with regulatory standards.
Approval or Further Studies
The drug may be approved for marketing, or the FDA may request additional studies to address outstanding concerns. Advisory committees may also weigh in on complex cases.
Step 5: Post-Market Monitoring
Approval is not the end of the drug development process. Once a drug enters the market, its safety continues to be monitored.
Key Activities
- Supplemental Applications: For changes in dosage, formulation, or indications.
- Generic Drug Approvals: Once patents expire, generic versions undergo bioequivalence testing.
- Active Surveillance: The FDA uses tools like the Sentinel Initiative to detect safety issues in real-time using electronic health records.
Emerging Trends in Drug Development

- AI and Machine Learning: These technologies enable the rapid identification of drug candidates and help to optimize clinical trial designs.
- Precision Medicine: Tailoring treatments based on genetic, environmental, and lifestyle factors is becoming increasingly common.
- Real-World Evidence (RWE): Post-market studies use real-world data to understand drug performance across diverse populations.
Conclusion
The drug development process is a dynamic journey driven by innovation, collaboration, and a commitment to patient safety. As the technology is being advanced, helps to improve our progress while following the industry standards. Integrating AI into medicine leads us to better treatment. With over 1.7 million new cases and 600,000 deaths each year, the life sciences sector must continue to innovate and improve the lives of people by using this process.







