Mallikaarjunan R, VP – regulatory services, Navitas Life Sciences
Mohammed Yaseen, senior regulatory associate, regulatory operations, Navitas Life Sciences
Prasana Santhuru, team lead, regulatory operations, Navitas Life Sciences
The pharmaceutical industry is characterized by a multitude of products with varying complexities and properties, each designed to improve human health and well-being. However, amidst this diversity, there has been a need for a standardized approach to identifying and documenting medicinal products across the globe. This imperative has led to the development of a shared framework by the International Organization for Standardization (ISO) known as the Identification of Medicinal Product (IDMP). This global standard strives to establish a common language for identifying, documenting, and exchanging information about medicinal products. By doing so, it not only elevates patient safety but also streamlines regulatory processes, thereby addressing a crucial need in the pharmaceutical landscape.
IDMP: An Overview
The IDMP framework comprises five distinct standards, each catering to different aspects of medicinal product identification and documentation:
- Medicinal Product Identification (MPID)
- Pharmaceutical Product Identifier (PhPID)
- Substance Identification (SubID)
- Dosage Form and Route of Administration
- Units of Measurement (UoM)
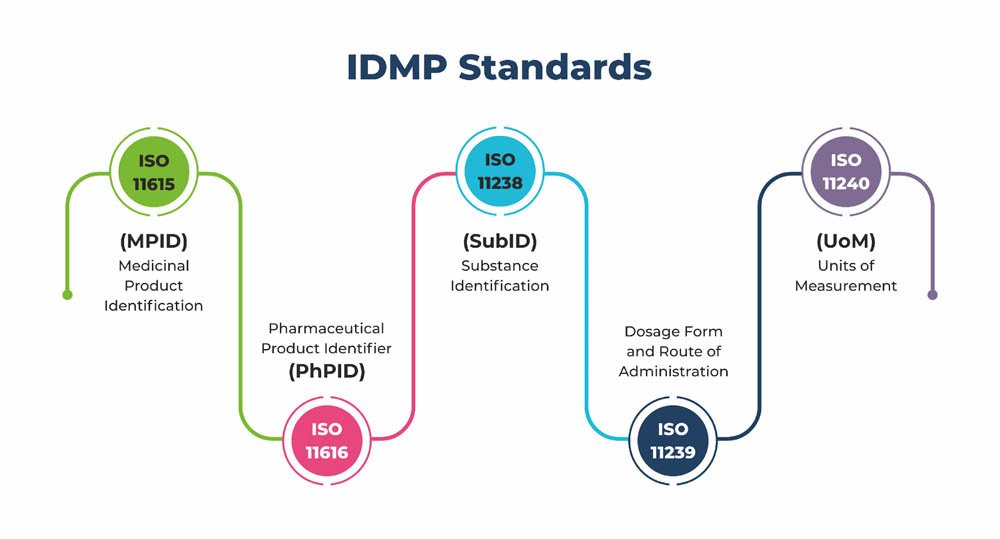
Together, these standards lay the foundation for a cohesive and harmonized approach to the description of medicinal products, from their constituents and properties to their manufacturing and regulatory particulars.
Benefits of IDMP Implementation
Regulatory Affairs – IDMP’s impact on Regulatory Affairs is considerable. By promoting the use of a standardized terminology and coding system, IDMP eliminates inconsistencies in regulatory processes. The implementation of IDMP has several benefits for Regulatory Affairs including:
- Improved Regulatory Compliance – IDMP helps to ensure that all stakeholders in the pharmaceutical supply chain, including manufacturers, distributors and regulatory authorities use the same terminology and coding system which helps to prevent inconsistence in regulatory process.
- Better Traceability – With IDMP, each medicinal product is assigned a unique identifier, which makes it easier to track the product throughout its lifecycle from development to post-marketing surveillance.
- Faster Approval Process – IDMP enables regulatory authorities to access information about medicinal products more quickly and easily which can speed the approval process and reduce the time to commercialize.
Pharmacovigilance – Unique identification of medicinal products helps in drastically improving pharmacovigilance. Harmonized definition of products also improves the quality of data sets used in safety signals, globally.
Clinical Trials – IDMP’s standardized data sets simplify the evaluation of medicines during clinical trials. Investigators can make more informed assessments, leading to improved understanding of drug effects and better decision-making.
Good Manufacturing Practice (GMP) – Manufacturing site inspections are streamlined with IDMP, as accessible and standardized data are presented in a defined format. This enhances transparency and efficiency in manufacturing processes.
Revised IDMP standards to improve description of medicinal products worldwide:
The ongoing revision of IDMP standards holds great promise for patients and the healthcare community. The updated standards will foster smoother information exchange between stakeholders and promote system interoperability within the medical field. By simplifying and unifying the exchange of information, these revisions are poised to revolutionize the way medicinal products are described and documented worldwide.
Key Aspects Covered by ISO IDMP Standards
ISO IDMP standards include a comprehensive array of aspects crucial for describing a medicinal product, like:
- Medicinal product name
- Ingredient substances
- Pharmaceutical product (route of administration, strength)
- Marketing authorization
- Clinical particulars
- Packaging
- Manufacturing
IDMP and XEVMPD: A Comparative Analysis
The European Union’s Extended EudraVigilance Medicinal Product Dictionary (xEVMPD) and IDMP share the common goal of enhancing drug safety and regulatory processes. However, they diverge in several key aspects:
| xEVMPD | IDMP | |
| Scope | EVMPD’s purpose is to collect the data of the medicinal products that are already existing or still in development. This collected information is used for evaluation of adverse reactions during the new drug development and for following the marketing authorizations of medicinal products within Europe | IDMP is a suite of five standards developed within the International Organization for Standardization (ISO) to facilitate the unique identification of medicinal products in the context of pharmacovigilance and the safety of medications throughout the world. |
| Database Geography | xEVMPD is a specific database for Europe | IDMP operates as a global database |
| Approval | It contains drug substance and drug product details that are approved in Europe only. | It contains drug substance and drug products details in brief which are approved word wide. |
| Extent of Information | It is focused on information related to marketing and surveillance of medicinal products. | It is more comprehensive and covers all aspects of medicinal product’s lifecycle from development to post authorization. |
| Purpose | It focuses on the submission of product information to the Eudravigilance system which is used specifically for the pharmacovigilance purpose | It is designed to facilitate the exchange of information between different regulatory authorities, enabling them to better coordinate their activities and share information on medicinal products. |
| Information Required | xEVMPD primarily requires information on the product’s name, dosage form as well as information about marketing authorization holder and packaging like SmPC/PIL. | It requires more detailed information including data on the product’s development, manufacturing, and quality control, as well as information on the product’s intended use and clinical data. |
IDMP Implementation in EMA and Beyond
The European Medicines Agency (EMA) has mandated the implementation of IDMP within the EU, with a gradual phased approach. The US, Canada, and Japan are also contemplating the adoption of IDMP as a global standard.
The first phase requires pharmaceutical companies to submit data on the identification of medicinal products, including information on organization and referential, product and substance information need to be mandatory in upcoming days.
The subsequent phases of IDMP implementation, which will cover the referential and regulatory activity domains, are expected to be implemented in the upcoming days, the specific timelines are yet to be confirmed by the EMA.
The publication of the EU Implementation Guidelines v2 in February 2021 started the 2-year implementation window. The EU IDMP Implementation Guide (EU IG) for the submission of data on medicinal products defines the implementation requirements of the ISO IDMP standards for the EU. The EU IG is the basis for submitting and exchanging medicinal product data in the EU.
The updated Chapter 7 of the EU IDMP Implementation Guide (EU IG), released on the EMA website on 18 January 2023, provides information on the approach followed by the European Medicines Agency (EMA) to enable transformation, migration and availability of authorized product data to the PMS database.
While a precise date for full IDMP implementation has not been announced yet by the EMA, it is noteworthy that the data migration process into the PMS software has been initiated, signifying a step forward in this crucial journey.
Challenges in IDMP Implementation
IDMP implementation has encountered delays due to various factors:
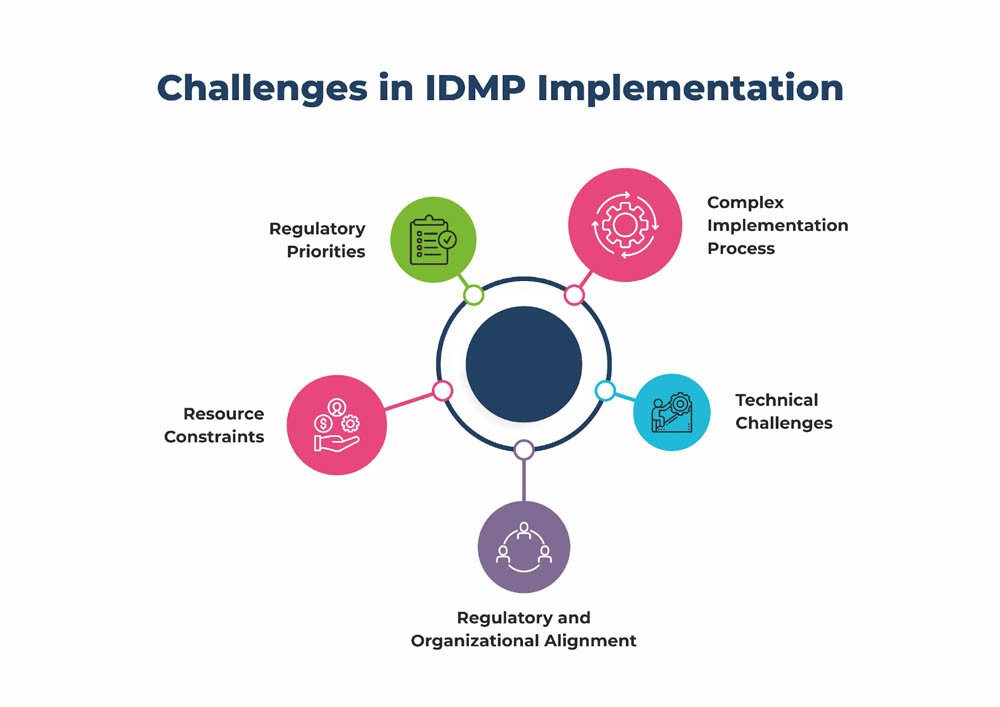
- Complex Implementation Process: IDMP implementation involves significant changes in data management systems and processes across the pharmaceutical industry. It requires coordination and collaboration between regulatory authorities, pharmaceutical companies, and other stakeholders. The complexity of implementing such a comprehensive system can contribute to delays.
- Technical Challenges: Implementing IDMP standards involves harmonizing and standardizing data across different databases and systems. This can be technically challenging, particularly for organizations with legacy systems or diverse data structures. Overcoming these technical challenges and ensuring interoperability can take time.
- Regulatory and Organizational Alignment: Different regulatory authorities may have varying timelines and approaches to implementing IDMP standards. Achieving alignment and consensus among these authorities can be a complex and time-consuming process. Additionally, individual organizations may require time to align their internal processes and systems with the new requirements.
- Resource Constraints: Implementing IDMP requires a significant investment of resources, including financial resources, skilled personnel, and technology infrastructure. Organizations may face resource constraints, which can slow down the implementation process.
- Regulatory Priorities: Regulatory authorities may have multiple priorities and initiatives that compete for attention and resources. IDMP implementation might be deprioritized or delayed due to other pressing regulatory concerns.
The transformative impact of the IDMP standard necessitates comprehensive change as an integrated process. These demand an understanding of regulations, recognizing process impacts, formulating remediation plans, and fostering a strategic vision. Evaluating improvements in data, systems, and processes is crucial, followed by a well-defined implementation strategy. This strategy should be based on the overarching vision as the compass that guides successful navigation towards a standardized, safer, and more efficient future.
Author Bios
Mallikaarjunan R
Malli is the VP – Regulatory Services, Navitas Life Sciences and has been in regulatory affairs/operations for 18+ years. His position and training in this field put him at the nexus of product lifelines dealing with end-to-end regulatory services (drug development, clinical studies, and post-marketing considerations). Malli currently manages the regulatory services group at Navitas Life Sciences and brings deep experience relating to developing strategies, continuous improvements, and resource plans.
Mohammed Yaseen
Mohammed Yaseen is a senior regulatory associate, regulatory operations, Navitas Life Sciences, and he has a post-graduate degree in Pharmacy (M.Pharmacy) . He has over 8 years of experience in the Pharmaceutical Industry (In Regulatory Affairs and Pharmacovigilance).
Prasana Santhuru S
Prasana Santhuru is a team lead, regulatory operations, Navitas Life Sciences, and has a master’s degree in pharmacy. He is a regulatory affair professional with 8+ Years of experience in dossier compilation, review, of Initial product registration and Post approval submissions with global regulatory authorities.
References:
- Identification of Medicinal Products (IDMP) | FDA
- What is Identification of Medicinal Products (IDMP)?, IDMP standards, medicinal products (freyrsolutions.com)
- Data on medicines (ISO IDMP standards): research and development | European Medicines Agency (europa.eu)
- ISO – Standards
- https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/products-management-services-implementation-international-organization-standardization-iso-standards_en.pdf
- https://www.europeanpharmaceuticalreview.com/article/144651/emas-new-guide-to-implementing-idmp-data-standards-triggers-the-countdown-to-change/
- https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/products-management-services-pms-implementation-international-organization-standardization-iso_en-0.pdf.
- https://www.ema.europa.eu/en/human-regulatory/overview/data-medicines-iso-idmp-standards-overview
10. Articles by EMA:
- “IDMP: A new era of global product identification” this article was published on EMA website provides and overview of IDMP and its importance in ensuring the safe use of medicinal products.
- “IDMP Implementation: A global view” This article published in the journal drug information association, discusses the challenges and benefits of implementing IDMP globally.
- “IDMP: A regulatory challenge for the pharmaceutical industry” – This article published in the journal of pharmaceutical Technology Europe, explores the impact of IDMP.
- “IDMP Implementation: A practical approach” – This article published in the journal regulatory focus, offer a practical guide to implementing IDMP, including the tips of managing data and ensuring compliance.
- “IDMP and Pharmacovigilance: The need for Harmonization” – This article published in the journal Drug Safety, discusses the role of IDMP in PV.







