In the field of biomedical research and imaging, fluorescence microscopy is a fundamental technology that enables researchers to see and analyze cellular and molecular structures with remarkable accuracy and detail. We explore the fundamentals, methods, uses, and most recent developments in fluorescence microscopy in this extensive book, highlighting the crucial role that this field plays in advancing scientific research and innovation.
Principles of Fluorescence Microscopy
Fluorescence microscopy relies on the phenomenon of fluorescence, where certain molecules, known as fluorophores, absorb light at a specific wavelength and emit light at a longer wavelength. This process enables the visualization of fluorescently labeled structures within biological samples, such as cells and tissues. Key components of a fluorescence microscope include an excitation light source, filters to select the excitation and emission wavelengths, and a detector to capture the emitted fluorescence. By selectively labeling target molecules with fluorescent probes, researchers can study their localization, dynamics, and interactions within living cells and organisms.
In addition to the basic principles outlined above, fluorescence microscopy offers versatility and adaptability, making it a powerful tool for a wide range of biological applications. The ability to selectively label specific molecules or structures within a sample provides researchers with invaluable insights into cellular processes, molecular interactions, and disease mechanisms.
Moreover, fluorescence microscopy can be combined with other imaging modalities, such as live-cell imaging, time-lapse microscopy, and super-resolution microscopy, to further expand its capabilities and address complex biological questions. Overall, the principles of fluorescence microscopy underpin its utility and significance in advancing our understanding of the intricate workings of the biological world.
Techniques in Fluorescence Microscopy
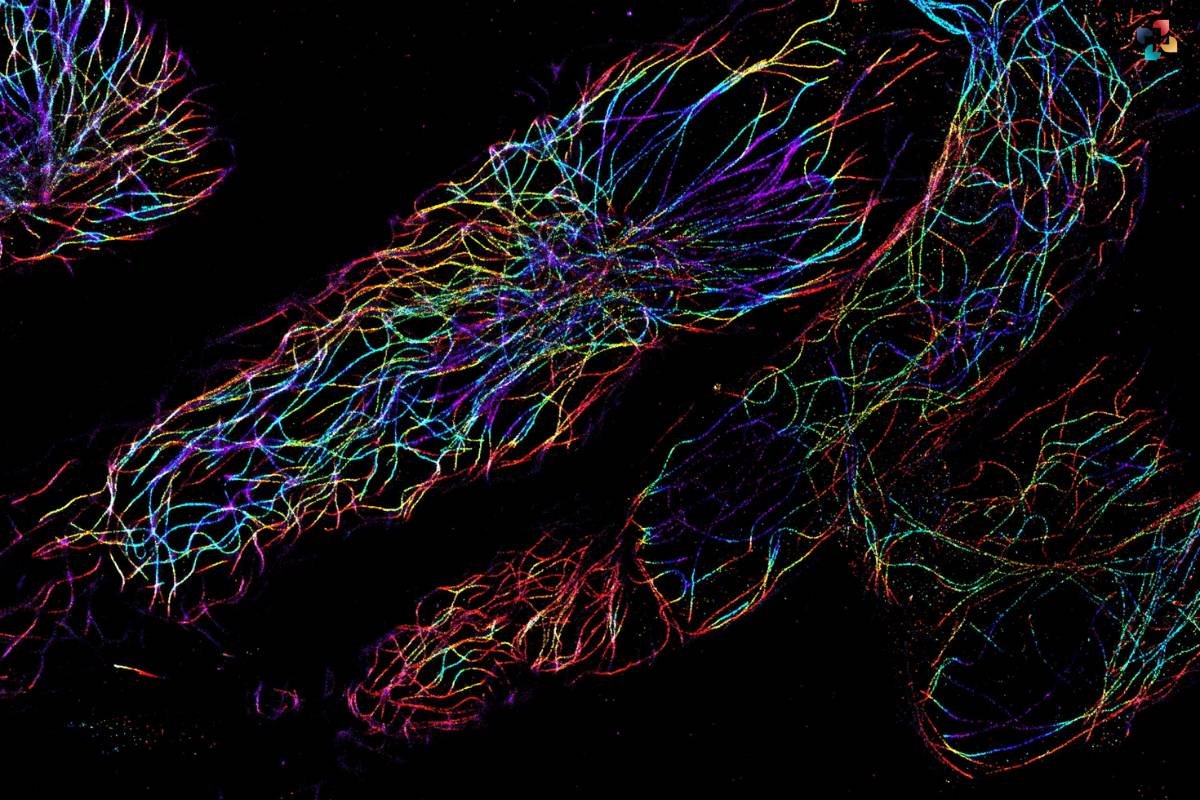
Various techniques have been developed to enhance the capabilities of fluorescence microscopy and address specific research questions. Confocal microscopy, for instance, utilizes a pinhole aperture to eliminate out-of-focus light, enabling optical sectioning and three-dimensional reconstruction of specimens. Super-resolution microscopy techniques, such as stimulated emission depletion (STED) microscopy and stochastic optical reconstruction microscopy (STORM), surpass the diffraction limit of light, achieving resolutions at the nanoscale level. Other specialized approaches, including fluorescence resonance energy transfer (FRET) and fluorescence lifetime imaging microscopy (FLIM), provide insights into molecular interactions and dynamics.
Applications of Fluorescence Microscopy
Fluorescence microscopy finds widespread applications across various fields of biology and medicine. In cell biology, it is employed to visualize subcellular structures, track cellular processes, and study protein localization and trafficking. In neuroscience, fluorescence microscopy enables the visualization of neuronal circuits, synaptic connections, and dynamic changes in neuronal activity.
In immunology and microbiology, it facilitates the detection and analysis of pathogens, immune responses, and host-pathogen interactions. Moreover, it plays a crucial role in drug discovery, diagnostics, and personalized medicine, aiding in the characterization of disease mechanisms and the development of therapeutic interventions.
Fluorescence microscopy offers unparalleled versatility and precision, making it indispensable in various scientific disciplines. Here are some additional applications and benefits:
1. Cancer Research
It is utilized to study cancer biology, including tumor growth, metastasis, and response to treatment. By visualizing cancer cells and their microenvironment, researchers can identify potential therapeutic targets and assess treatment efficacy.
2. Genetics and Genomics

Fluorescence microscopy plays a crucial role in genetic and genomic studies, allowing for the visualization of DNA, RNA, and chromatin dynamics. It enables researchers to investigate gene expression, genome organization, and epigenetic modifications.
3. Developmental Biology
It is instrumental in studying embryonic development, tissue morphogenesis, and organogenesis. By labeling specific developmental markers, researchers can track the differentiation and migration of cells during embryogenesis.
4. Biomedical Imaging
In clinical settings, it is used for diagnostic purposes, such as identifying pathogens in infectious diseases and detecting biomarkers in tissue samples. It offers high sensitivity and specificity, enabling accurate disease diagnosis and prognosis.
5. Nanotechnology and Material Science
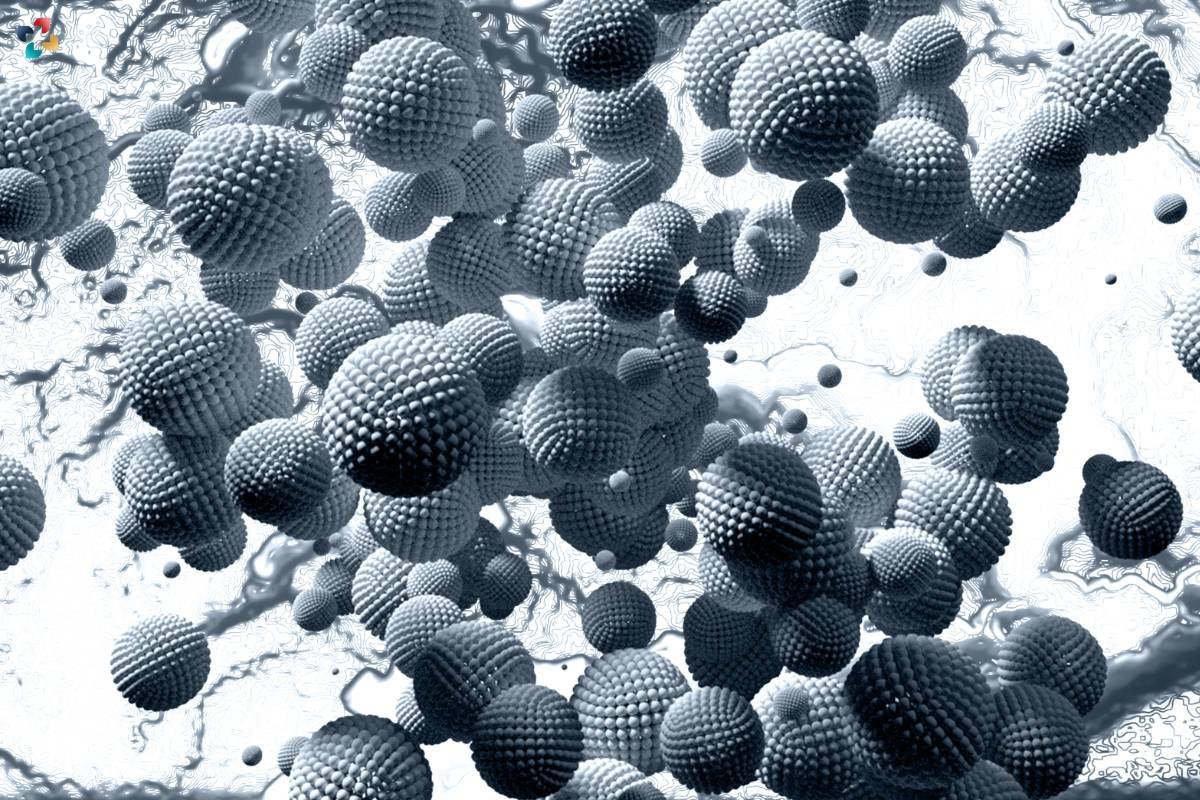
It is employed in nanotechnology and material science to characterize nanoparticles, nanomaterials, and biomaterials. It allows for the visualization of molecular interactions and structural properties at the nanoscale, facilitating the design and optimization of novel materials for various applications.
Overall, it continues to drive advancements in research and technology, offering invaluable insights into the complexities of biological systems and paving the way for innovative discoveries and solutions in healthcare and beyond.
Advancements in Fluorescence Microscopy
Fluorescence microscopy has advanced recently, pushing the limits of imaging capabilities and broadening the field of biological research. Enhancements in sensitivity, resolution, and speed have resulted from advancements in light sources, detectors, and imaging modalities. The efficiency and depth of analysis are increased when numerous molecular targets can be simultaneously visualised within a single sample thanks to multiplexed imaging techniques. Additionally, improvements in sample preparation methods, such as tissue clearance and expansion microscopy, allow for high-fidelity imaging of bigger and more complex biological specimens.
FAQs:
1. What is the difference between fluorescence microscopy and traditional light microscopy?
Fluorescence microscopy utilizes fluorescent labels to selectively visualize specific molecules or structures within biological samples, offering enhanced sensitivity and specificity compared to traditional light microscopy, which relies on transmitted or reflected light.
2. How are fluorescent labels introduced into biological samples for imaging?
Fluorescent labels, also known as fluorophores or fluorescent probes, can be introduced into biological samples through various methods, including chemical staining, genetic encoding (e.g., fluorescent proteins), and antibody-based labeling techniques.
3. What are some common fluorophores used in fluorescence microscopy?
Common fluorophores used in fluorescence microscopy include fluorescein isothiocyanate (FITC), rhodamine, cyanine dyes (Cy3, Cy5), green fluorescent protein (GFP), and red fluorescent protein (RFP), among others.
4. Can fluorescence microscopy be used for live-cell imaging?
Yes, fluorescence microscopy is widely used for live-cell imaging, enabling the visualization of dynamic processes and interactions within living cells and organisms. Specialized microscopy setups, such as spinning disk confocal and total internal reflection fluorescence (TIRF) microscopy, are often employed for high-speed and high-resolution live-cell imaging.
5. What are the limitations of fluorescence microscopy?
While fluorescence microscopy offers numerous advantages for biological imaging, including high sensitivity and specificity, it also has limitations such as photobleaching (loss of fluorescence signal over time), phototoxicity (damage to biological samples caused by exposure to light), and limited penetration depth in thick or opaque specimens. Advanced imaging techniques and strategies are continually being developed to overcome these challenges and push the boundaries of fluorescence microscopy.