Key Points:
- WHO weight loss injections are recommended for adults with obesity as part of long-term care.
- Injections work alongside diet and exercise to control appetite and blood sugar.
- Access is limited, and guidance for children and adolescents will be released soon.
The World Health Organization conditionally recommended the use of WHO weight loss injections for treating obesity on Dec. 1, marking the first time the health agency has formally supported such medication. The guidance applies to adults with a Body Mass Index of 30 or higher and excludes pregnant women. WHO officials said the decision reflects growing evidence that obesity is a chronic condition requiring long-term care.
New Guidance Targets Global Obesity Rise
WHO Director-General Tedros Adhanom Ghebreyesus said the recommendation “recognises that obesity is a chronic disease that can be treated with comprehensive and lifelong care.” He added that medication cannot solve obesity alone, but can support millions facing the condition and reduce related health risks.
The agency estimates that more than one billion people currently live with obesity worldwide. It projects that the number will rise to two billion by 2030. The new recommendation addresses this escalating trend and sets out the first formal advice on the long-term use of WHO weight loss injections.
How the Therapies Work
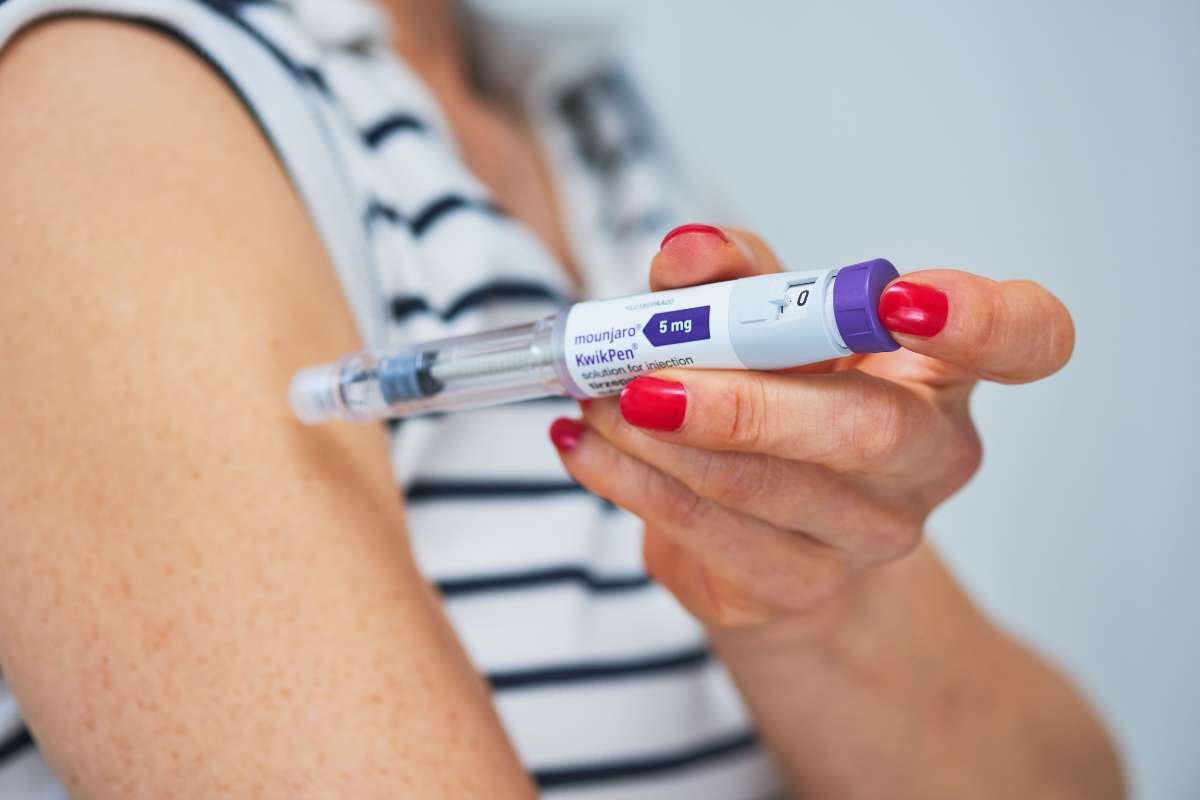
The injections, known as GLP-1 therapies, work by mimicking a hormone that increases insulin release, regulates blood sugar, and reduces appetite. The guidance covers three drugs: semaglutide, sold under names including Ozempic and Wegovy; tirzepatide, available as Mounjaro and Zepbound; and liraglutide, an older treatment from the same class.
The first recommendation advises these medicines as part of long-term obesity treatment in adults. The second highlights the importance of offering interventions such as a healthy diet and physical activity alongside medication.
Access Challenges Ahead
WHO officials said access remains a significant barrier. Even with expanded production, the agency expects GLP-1 therapies to reach fewer than 10% of people who could benefit by 2030. High demand and limited supply continue to affect their availability in many regions.
The move follows decisions in several countries, including the United Kingdom, where regulators licensed Wegovy as a weight loss drug and made it available through the National Health Service (NHS). Mounjaro was also expected to be accessible through GP surgeries and community services beginning June 23.
Implementation Varies Across the UK
However, access across England has been uneven. As of early August, only eight of 42 NHS Integrated Care Boards had begun offering the treatment. Many other boards said they could not confirm when services would start.
In August, the National Institute for Health and Care Excellence (NICE) urged providers to offer structured advice and follow-up support to patients using WHO weight loss injections, including at least a year of monitoring to help prevent weight regain. The guidance includes support to build long-term behavioural habits, use self-monitoring tools, and rely on broader resources ranging from online communities to family and local activities.
More Guidance Expected for Younger Patients
During a conference call, WHO officials said they would release separate guidance next week on treating obesity in children and adolescents. The organisation noted that early intervention will be important as global obesity rates continue to rise.
The latest recommendations add new direction to the global response to obesity and outline how WHO weight loss injections can be used alongside lifestyle measures to improve long-term outcomes.