The intricate dance within the nucleus of a cell, choreographed by histones, creates a symphony of cellular activities that govern vital processes such as gene expression, DNA repair, and disease pathways. In this exploration, we unravel the mysteries of histones, the conductors orchestrating the harmony within chromatin. Histones, like skilled musicians, play a pivotal role in creating a harmonious environment where the vast stretches of DNA are packed, organized, and regulated. Understanding the nuances is essential for deciphering the complex language of chromatin and its profound impact on cellular function.
Unraveling the Mysteries of Histones:
1. The core of Chromatin Architecture
Histones serve as the foundational building blocks of chromatin, the dynamic structure that packages and protects DNA within the cell nucleus. The core histones – H2A, H2B, H3, and H4 – assemble to form an octameric structure known as the nucleosome, around which DNA elegantly winds. This structural foundation not only compacts DNA but also provides accessibility to regulatory elements, influencing gene expression. The nucleosome structure is akin to beads on a string, with histones acting as anchors shaping the chromatin landscape.
2. Nucleosome Remodeling and Histone Variants
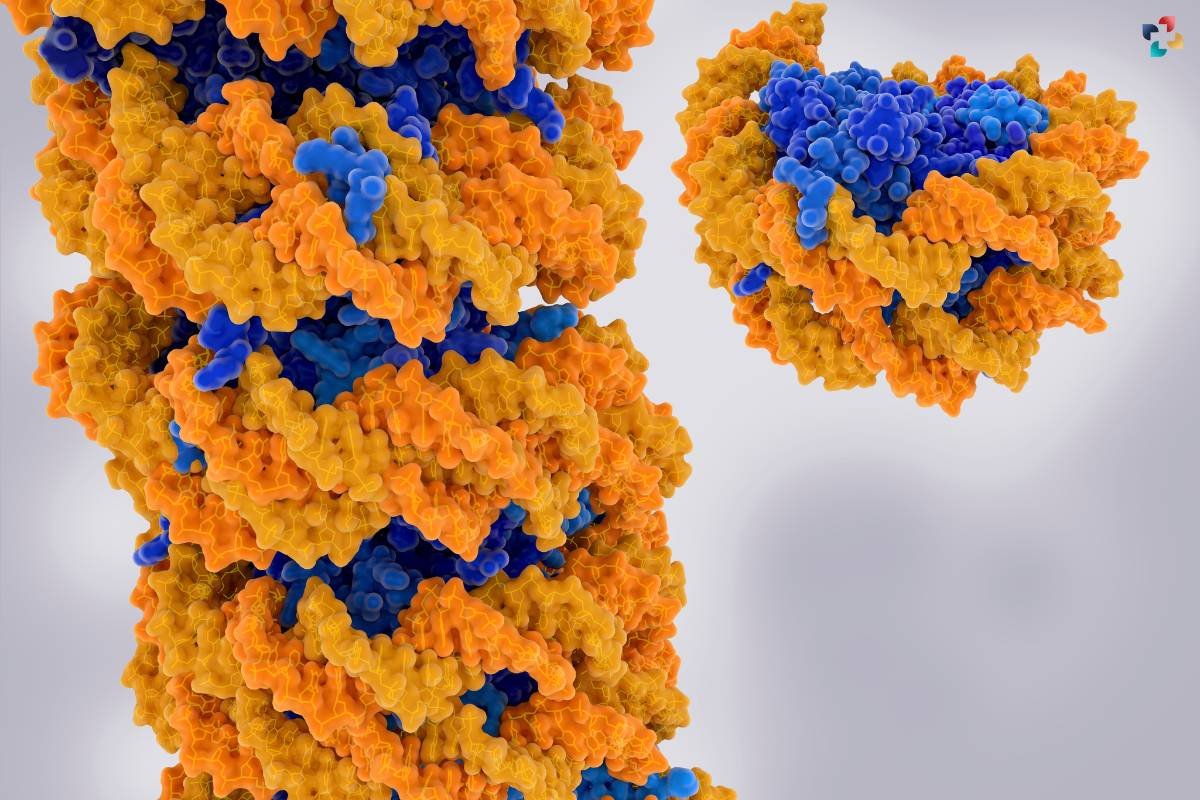
Nucleosome remodeling, a crucial aspect of DNA repair mechanisms, involves altering the positioning of histones to allow repair factors access to damaged DNA. This process facilitates essential mechanisms such as nucleotide excision repair and homologous recombination, ensuring the integrity of the genome. Furthermore, histone variants like H2A.X act as beacons, selectively incorporating into chromatin at sites of DNA damage. These variant histones play a signaling role, orchestrating the recruitment of repair machinery, and emphasizing their importance in maintaining genome stability.
3. Histone Modification
The Epigenetic Symphony: Histones undergo a myriad of post-translational modifications that collectively form the epigenetic symphony. Acetylation and methylation are among the most studied modifications, with acetylation often associated with transcriptional activation and methylation displaying context-dependent effects. Phosphorylation of histones, a dynamic process linked to cell cycle regulation and DNA damage response, adds another layer to the symphony. Ubiquitination contributes to histone turnover and plays a role in orchestrating chromatin structure.
4. Readers, Writers, and Erasers
The dynamic landscape of histone modifications is regulated by a trio of protein players – writers, erasers, and readers. Enzymes act as writers by adding modifications, erasers remove modifications, and reader proteins recognize specific modifications, interpreting the epigenetic code. This intricate network of proteins ensures the precise regulation of chromatin dynamics, allowing cells to finely tune gene expression in response to various stimuli.
5. Relationship with Gene Expression
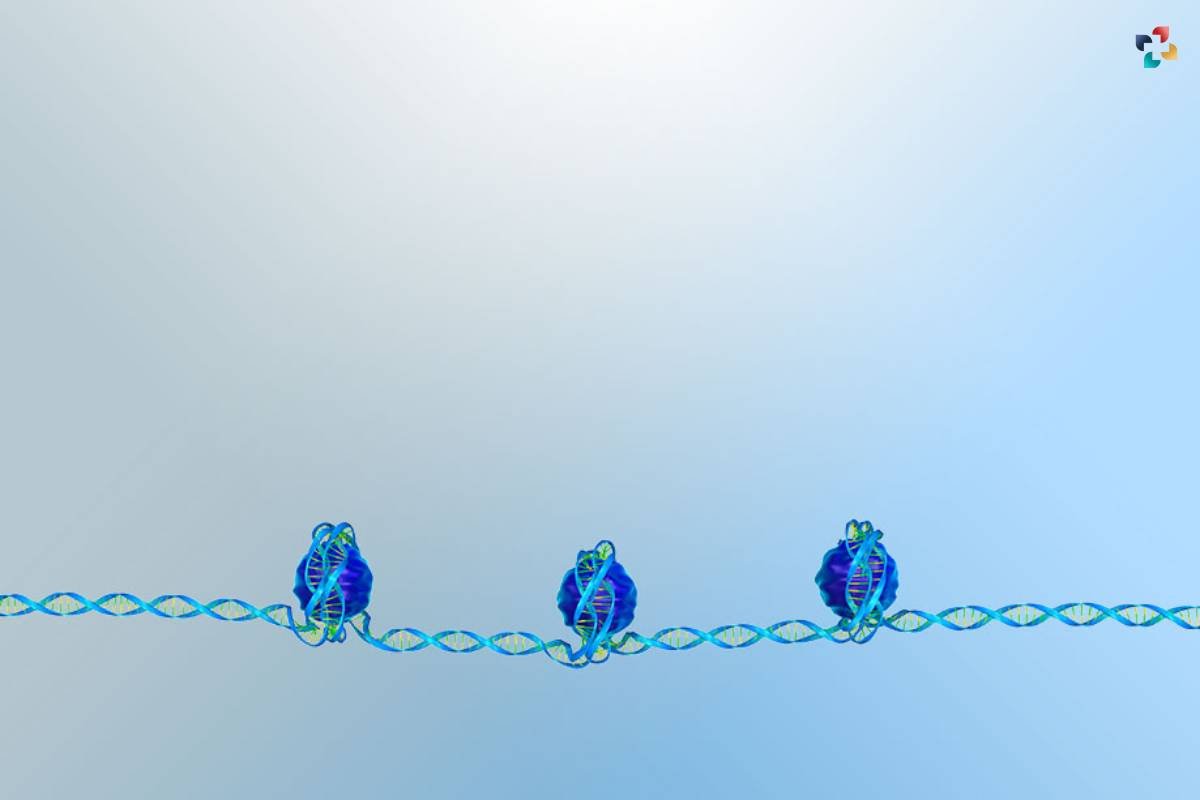
A Molecular Ballet: Histones play a central role in the regulation of gene expression, acting as key choreographers in the molecular ballet within the nucleus. Chromatin structure, influenced by histone modifications, dictates the accessibility of DNA to the transcriptional machinery. Acetylation, for instance, often relaxes chromatin, facilitating gene activation. Conversely, certain modifications, such as methylation of histone H3 at specific lysine residues, contribute to gene silencing and repression. This delicate balance in histone modifications orchestrates a molecular ballet, ensuring precise regulation of gene expression essential for cellular function.
6. Epigenetic Inheritance
Beyond individual gene regulation, histones contribute to epigenetic inheritance – the passage of modifications from one generation of cells to the next. This phenomenon forms a cellular memory, influencing cell fate and development. The heritability of histone modifications adds a layer of complexity to cellular regulation, allowing cells to retain information about their past experiences and adapt accordingly.
7. Dynamics of Histone Exchange
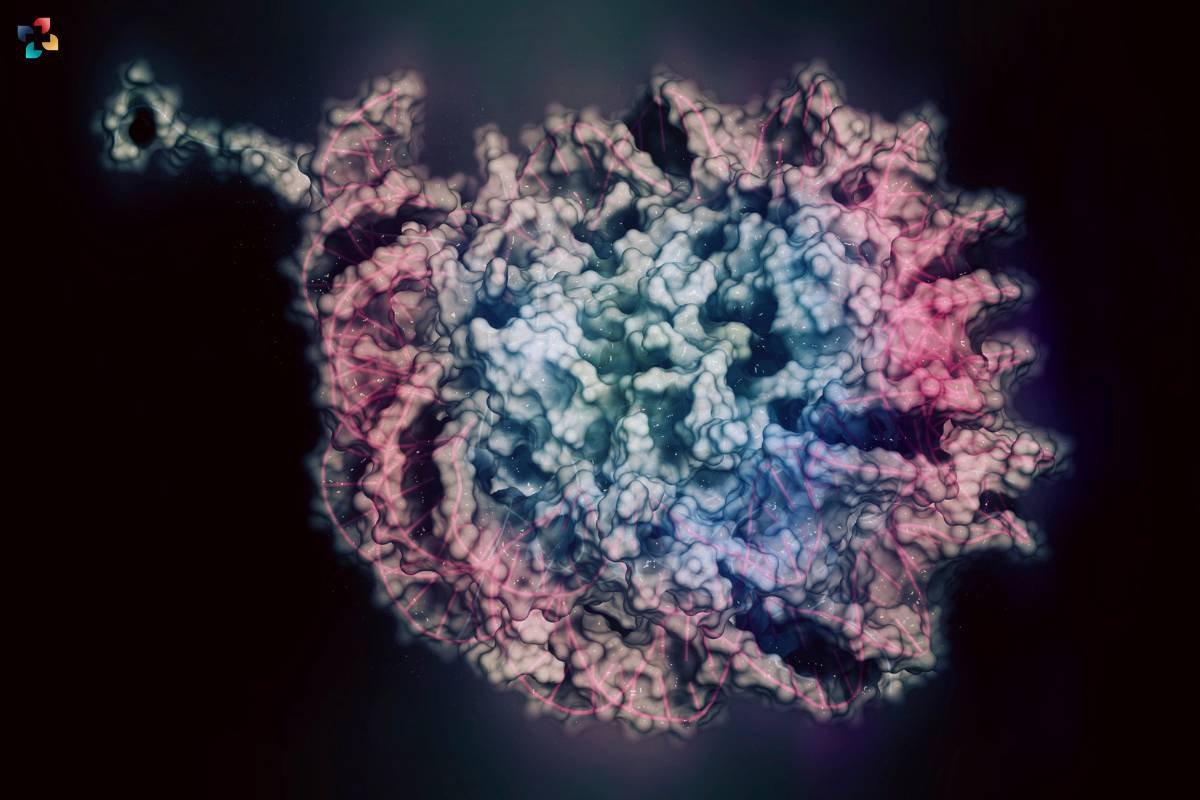
Balancing Stability and Plasticity: While core histones are often considered stable, cells undergo continuous histone turnover. New histones are synthesized and incorporated into chromatin, contributing to the dynamic nature of chromatin structure. The incorporation of histone variants further adds complexity to chromatin dynamics, selectively deposited into specific genomic regions, influencing processes such as transcriptional regulation and DNA repair. This continuous exchange of histones and their modifications contribute to the plasticity of chromatin, allowing cells to adapt to environmental cues, developmental signals, and changes in cellular state.
8. Emerging Frontiers
Histones in Disease and Therapeutics: Dysregulation of histone modifications is implicated in various diseases, including cancer and neurodegenerative disorders. Aberrant histone modifications can lead to altered gene expression patterns, contributing to disease progression. The recognition of histones’ pivotal role in disease has spurred the development of targeted therapies. Histone deacetylase (HDAC) inhibitors, for example, are being explored as potential anticancer agents, aiming to restore normal chromatin function in diseased cells. The evolving field of epigenetic editing, utilizing technologies like CRISPR-based systems, holds promise for precisely manipulating histone modifications for therapeutic purposes.
9. Challenges and Future Directions
Navigating the Histone Code: Decoding the histone code, the intricate combination of histone modifications remains a complex language that scientists are deciphering. Understanding the context-specific effects of different modifications and their cross-talk is crucial for unraveling the histone code. Advances in technology, such as high-throughput sequencing and mass spectrometry, have revolutionized the study of histones, providing a comprehensive view of histone modifications across the genome. However, ethical considerations regarding the potential manipulation of histones for therapeutic purposes underscore the need for responsible use of emerging technologies.
Conclusion: Orchestrating the Chromatin Symphony:
In the complex symphony of cellular activities, histones play the crucial role of conductors, arranging the chromatin structure that determines how cells function. Histones become indispensable components of the molecular ballet of life, from their structural role in nucleosomes to their dynamic engagement in gene expression, DNA repair, and disease pathways.
The ongoing investigation into the world of histones offers new perspectives on the underlying ideas that control the complex dance of chromatin and its effects on both sickness and health in cells. As our understanding of histones grows, the goal of scientific research remains focused on deciphering the subtleties of the histone code and leveraging this knowledge for therapeutic interventions, ensuring a harmonious balance within the cellular symphony.







