For millennia, people have been fascinated by and curious about the complexities of human genetics. Single-gene disorders are distinct from other genetic variants in that they are brought on by mutations in a single gene. These conditions, which are frequently uncommon but significant, can have a significant impact on a person’s health and well-being. We go into the world of single-gene illnesses in this thorough examination, looking at their traits, frequency, and the intriguing tales they tell about the relationship between genes and health.
Understanding Single-Gene Disorders: Unraveling the Genetic Code
1. Defining Single-Gene Disorders
Single-gene disorders, also known as monogenic disorders, result from mutations in a single gene. Unlike complex disorders influenced by multiple genes and environmental factors, these conditions are directly linked to alterations in a specific gene’s structure or function.
2. Types of Genetic Mutations
The mutations responsible for single-gene disorders can take various forms, including point mutations, insertions, deletions, and chromosomal abnormalities. Each type of mutation can have distinct effects on the gene’s normal function, leading to a spectrum of disorders.
3. Mendelian Inheritance Patterns
Understanding single-gene disorders often involves grasping Mendelian inheritance patterns, which include autosomal dominant, autosomal recessive, and X-linked inheritance. These patterns dictate how a mutated gene is passed from one generation to the next.
Examples of Single-Gene Disorders: A Glimpse into Genetic Variability
1. Cystic Fibrosis (CF)
Cystic Fibrosis, an autosomal recessive disorder, is caused by mutations in the CFTR gene. This gene encodes a protein essential for regulating the flow of salt and fluids in and out of cells. Mutations result in thick, sticky mucus, impacting the respiratory, digestive, and reproductive systems.
2. Huntington’s Disease
An autosomal dominant disorder, Huntington’s disease is characterized by the progressive degeneration of nerve cells in the brain. The mutation responsible is found in the HTT gene, leading to the production of a faulty protein. Individuals with a single copy of the mutated gene are affected.
3. Sickle Cell Anemia
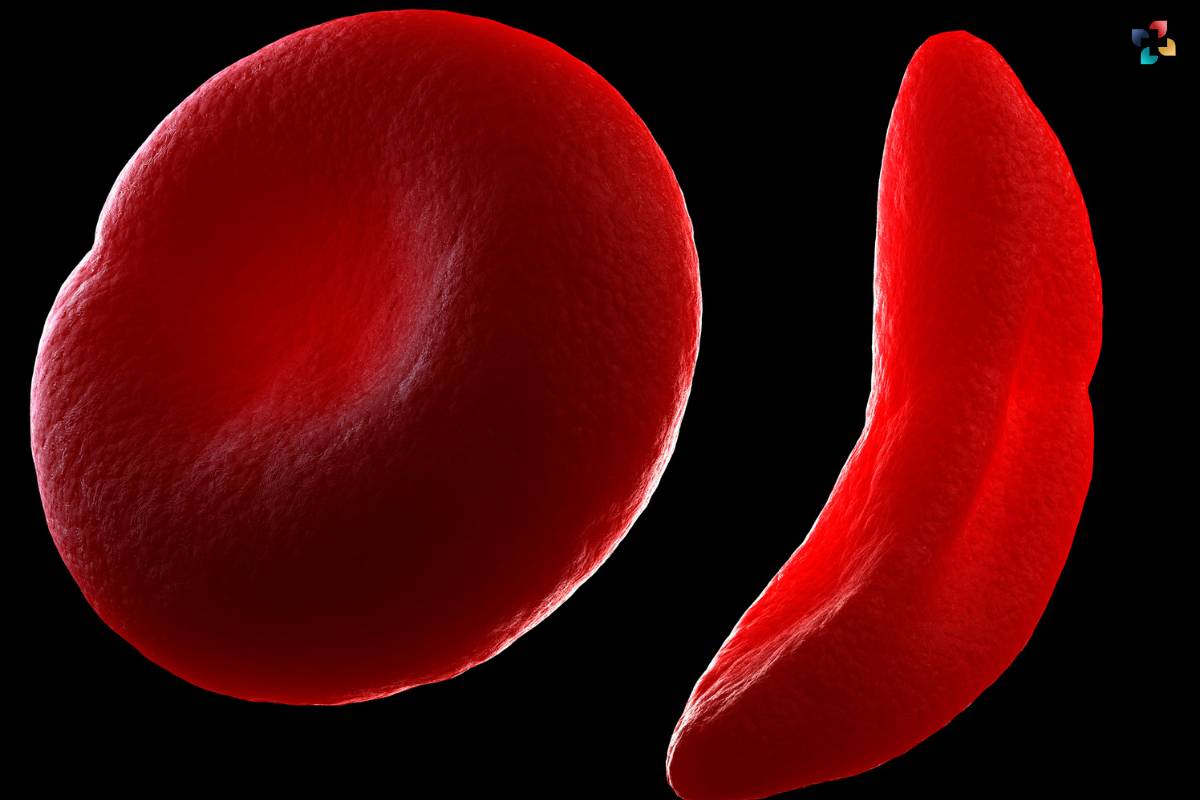
Sickle Cell Anemia is an autosomal recessive disorder caused by mutations in the HBB gene, leading to the production of abnormal hemoglobin. This results in the characteristic sickle-shaped red blood cells, causing pain, anemia, and various complications.
4. Duchenne Muscular Dystrophy (DMD)
Duchenne Muscular Dystrophy, an X-linked recessive disorder, arises from mutations in the DMD gene. This gene provides instructions for producing a protein essential for muscle structure. Mutations lead to progressive muscle degeneration and weakness.
5. Hemophilia A
Hemophilia A, an X-linked recessive disorder, is caused by mutations in the F8 gene, which encodes a clotting factor. Individuals with hemophilia A experience prolonged bleeding and are prone to excessive bleeding after injuries or surgeries.
6. Phenylketonuria (PKU)
Phenylketonuria, an autosomal recessive disorder, results from mutations in the PAH gene, leading to the inability to break down phenylalanine. Accumulation of phenylalanine can cause intellectual disabilities and other neurological issues.
Also Read: Understanding and Managing Autoimmune Disorders
Genetic Testing and Diagnosis: Illuminating the Genetic Blueprint
1. Prenatal Genetic Testing
Advancements in genetic testing have enabled the identification of single-gene disorders during pregnancy. Techniques such as chorionic villus sampling (CVS) and amniocentesis allow for the examination of fetal DNA, providing insights into potential genetic conditions.
2. Carrier Screening
Carrier screening, a preconception or early pregnancy test, helps individuals assess their risk of passing on genetic disorders to their children. This is particularly relevant for autosomal recessive conditions where both parents must be carriers for the disorder to manifest.
3. Predictive Genetic Testing
Predictive genetic testing allows individuals with a family history of a specific single gene disorder to determine their risk of developing the condition. This type of testing is often associated with late-onset disorders, such as Huntington’s disease.
Research and Therapeutic Advances: Charting the Future of Genetic Medicine
1. Gene Therapy
The field of gene therapy holds promise for addressing single-gene disorders. Experimental approaches involve introducing functional genes into cells to replace or compensate for defective ones. Ongoing research aims to refine gene therapy techniques for a range of genetic conditions.
2. CRISPR-Cas9 Technology
CRISPR-Cas9 technology has revolutionized gene editing, offering precise tools to modify specific genes. While still in the early stages, CRISPR holds the potential for correcting genetic mutations responsible for single-gene disorders, paving the way for targeted therapeutic interventions.
3. Personalized Medicine
Advancements in understanding the genetic basis of diseases have fueled the emergence of personalized medicine. Tailoring treatments based on an individual’s genetic makeup holds the potential to optimize therapeutic outcomes, especially in the realm of single-gene disorders.
Challenges and Ethical Considerations: Navigating the Genetic Frontier
1. Limited Treatment Options
While scientific strides are being made, many single-gene disorders still lack definitive treatments. The rarity and complexity of these conditions pose challenges in developing targeted therapies for each specific disorder.
2. Genetic Counseling

Genetic counseling plays a crucial role in helping individuals and families navigate the complexities of single-gene disorders. Providing informed decision-making support, genetic counselors help individuals understand their risks, make choices about testing, and cope with the implications of genetic conditions.
3. Ethical Considerations in Genetic Manipulation
As technologies like CRISPR-Cas9 advance, ethical considerations surrounding genetic manipulation become increasingly complex. Balancing the potential benefits of gene editing with concerns about unintended consequences and unforeseen ethical dilemmas is an ongoing challenge.
Conclusion: Deciphering the Genetic Tapestry of Health
The genetic code displays the intricate workings of human biology as well as the difficulties that result when mutations upset the careful balance in the interesting field of single-gene disorders. The tales of these genetic abnormalities act as lighthouses directing scientists, medical professionals, and people toward knowledge, prevention, and treatment as science and medicine progress.
The field of genetic medicine is changing, from the accuracy of genetic testing in diagnosis to the revolutionary promise of new treatments. While the road ahead may be marked by challenges and ethical considerations, the pursuit of unraveling the mysteries encoded in our genes holds the promise of a future where the impact of single-gene disorders is mitigated, and individuals can navigate their genetic destinies with newfound knowledge and empowerment.