[Source-news-medical.net]
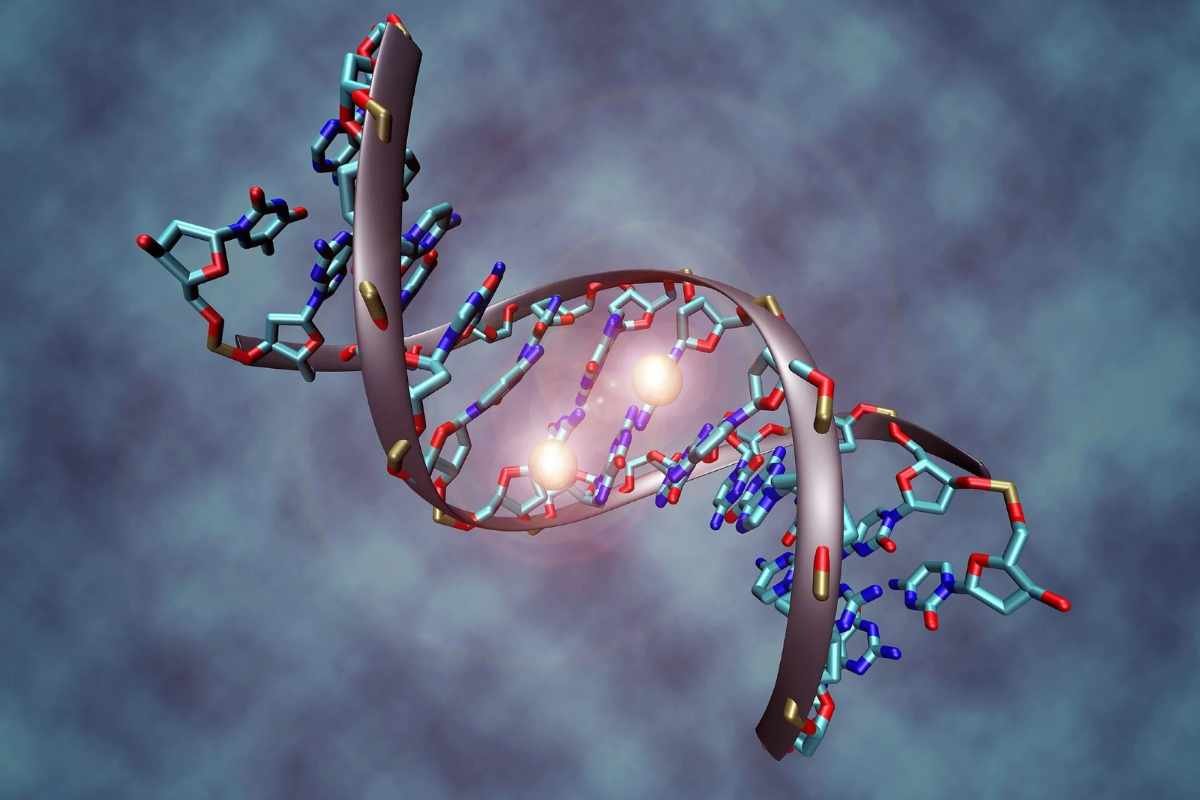
Recent research from scientists in Germany has uncovered a critical mechanism by which astrocytes, a type of brain cell, can transform into neural stem cells, revealing new avenues for brain repair. Published in the journal Nature, the study explores how ischemic injury (restricted blood flow) induces astrocytes to modify their DNA methylation patterns, shifting them toward a stem cell-like state. This transformation involves the methylation of astrocyte-specific genes and the demethylation of genes associated with stem cells, a process controlled by the enzyme DNA methyltransferase 3 alpha (DNMT3A).
This finding aligns with earlier discoveries that neural stem cells (NSCs) in the adult mammalian brain can be activated to produce new neurons, especially following injury. The study employed cutting-edge techniques, such as single-cell nucleosome, methylome, and transcriptome sequencing (scNMT-seq), to analyze how changes in DNA methylation affect gene expression in these cells. The results suggest that astrocytes in the striatum, a brain region traditionally not associated with neurogenesis, can acquire neurogenic potential under certain conditions, highlighting a latent capacity for neural repair and regeneration.
Methodology and Data Analysis
The study used male mice of various genetic backgrounds, including C57BL/6N and TiCY lines, to investigate the molecular mechanisms underlying astrocyte reprogramming. Researchers induced ischemia by occluding the bilateral common carotid artery and then conducted single-cell suspensions and fluorescence-activated cell sorting to isolate different brain cell populations. They employed a miniaturized scNMT-seq protocol to profile both the transcriptome and epigenome of single cells, while gene knockout techniques were used to assess the role of DNMT3A in these processes.
Data from these experiments were analyzed using various bioinformatics tools, such as Seurat and MethSCAn, to evaluate differential methylation and gene expression. The researchers identified distinct methylation patterns between dormant (qNSC1) and active NSCs (qNSC2), revealing that astrocytes resembling qNSC1 could transition to a more stem-cell-like state (qNSC2) under ischemic conditions. The study found that DNA methylation played a crucial role in silencing or activating gene expression, depending on its location relative to the transcription start site (TSS).
Implications and Future Directions
The study’s findings offer significant insights into the dynamic role of DNA methylation in brain cell plasticity. After ischemic injury, astrocytes in both the vSVZ and striatum adopted a methylation profile characteristic of NSCs, leading to increased neurogenesis. Interestingly, while most astrocytes reverted to their original state after 21 days, a subset retained a stem cell-like methylome or showed only partial reversion. This suggests a more complex dynamic of methylation changes post-injury, with interferons playing a key role in establishing specific DNA methylation regions required for astrocyte reprogramming.
The research also highlighted the essential role of DNMT3A in neurogenesis. In mice lacking this enzyme, the generation of new neurons following ischemia was significantly impaired, indicating that DNMT3A-mediated methylome remodeling is vital for activating the neurogenic potential of astrocytes. These findings open up new therapeutic possibilities, suggesting that manipulating DNA methylation could enhance the brain’s capacity for repair and regeneration. Future research could explore the potential of targeted methylation modification as a strategy for treating neurological disorders and even certain cancers.
- Did you find this news helpful? Visit more of our blogs! The Lifesciences Magazine