Source-News-Medical
The efficacy of a senolytic therapy based on chimeric antigen receptor (CAR) T-cells is assessed in a new study published in Nature Ageing. Senolytic CAR T-cells are used in this therapy to specifically target urokinase plasminogen activator receptor (uPAR)-positive cells, which proliferate with age.
Context
Stress causes an irreversible cell cycle arrest known as cellular senescence. Senescence-associated secretory phenotype (SASP) refers to the production of pro-inflammatory cytokines and matrix remodelling enzymes under stressful situations.
SASP helps draw in immune cells that support tissue repair and senescent cell elimination in young people with physiological circumstances like wound healing and tumour suppression. Senescent cells proliferate in older people because their immune systems are less effective and their tissue damage is greater.
Until yet, the majority of senoylatic therapy have consisted of small-molecule medications that are poorly targeted to the afflicted region and need to be administered repeatedly. In contrast, a single target antigen that is expressed differently in CAR T-cells than in normal tissues is needed. Furthermore, CAR T-cells are “living drugs” that can be injected once and continue to work for years, mediating their effects.
Concerning the study
Senescent cell depletion has been demonstrated by CAR T-cells to target the cell-surface protein uPAR. In order to control health span, the current study investigates whether CAR T-cells can safely and effectively eliminate senescent cells in aged mice.
The study included mice of both sexes, ages 8–12 weeks and 18–20 months. They were housed in communal quarters free from pathogens.
Standard conditions for temperature and humidity were maintained, and a 12-hour light/dark cycle was employed. A conventional diet was consumed by ageing animals, while a high-fat diet (HFD) was consumed by a subset of mice.
Red blood cells were lysed after livers were separated and filtered for flow cytometry examination. A single-cell RNA sequencing study was conducted, and then mouse T-cell growth, isolation, and transduction were carried out. Post-mortem examination of several organs was carried out, and complete blood measures were recorded.
The Potential of CAR T-cell Therapy in Combination With Antibodies for the Treatment of All
Important conclusions
It has been demonstrated that senolytic cell therapies can lessen physiological aging-related symptoms, such as metabolic dysfunction. Therefore, both immune and non-immune uPAR-positive cells probably contribute to the senescence load in old tissues, since the proportion of uPAR-positive cells tends to increase with age.
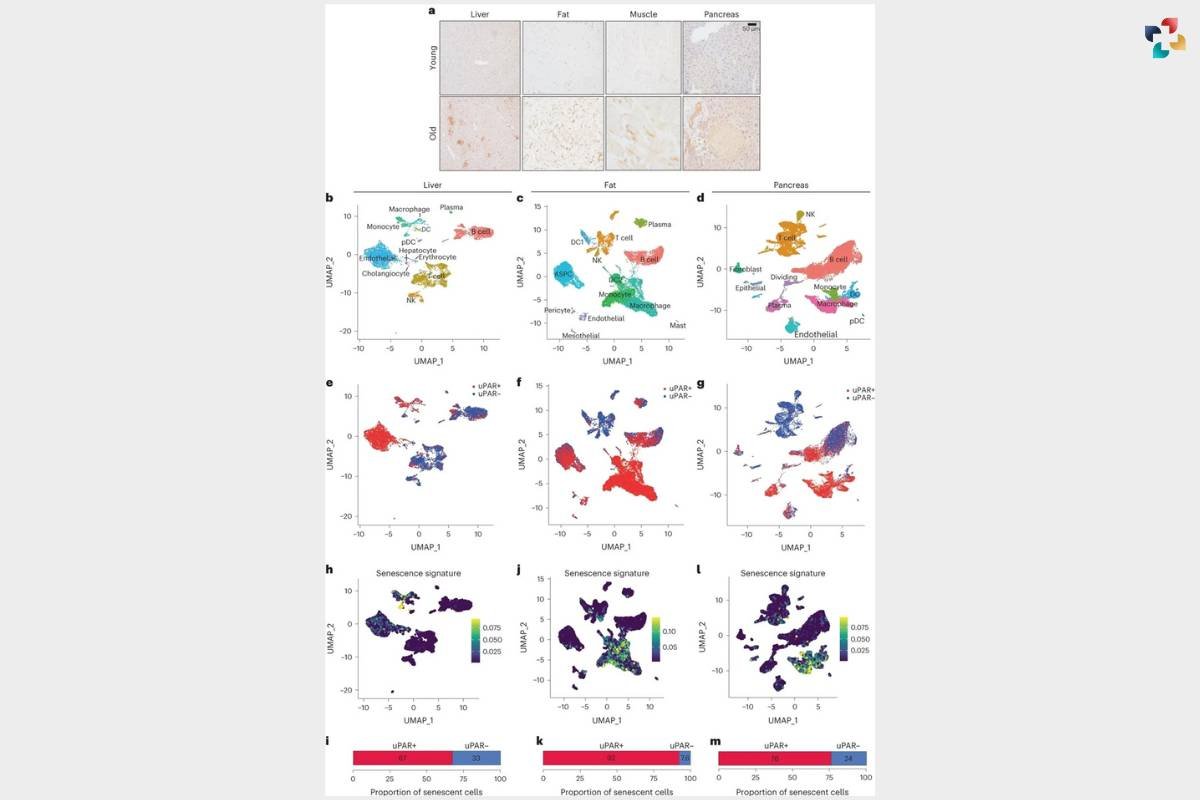
a, Immunohistochemical staining of mouse uPAR in liver, adipose tissue, muscle and pancreas from young (age 3 months) or old (age 20 months) mice (n = 3 per age). b–m, Single-cell analysis of uPAR expression and senescence. uPAR-positive and uPAR-negative cells were sorted from the liver, adipose tissue and pancreas of 20-month-old mice and subjected to single-cell RNA-seq by 10x chromium protocol (n = the sequencing of four mice where two females were combined into one replicate and two males were combined into another replicate).
b, Uniform manifold approximation and projection (UMAP) visualization of liver cell types.
c, UMAP visualization of adipose tissue cell types.
d, UMAP visualization of pancreas cell types.
e, UMAP visualization of hepatic uPAR-negative and uPAR-positive cell types.
f, UMAP visualization of adipose uPAR-negative and uPAR-positive cell types.
g, UMAP visualization of pancreatic uPAR-negative and uPAR-positive cell types.
h,j,l, UMAP visualizations with senescence signature scores24 in each cell indicated by the color scale.
i,k,m, Quantification of the proportion of uPAR-positive and uPAR-negative cells contributing to the respective senescence signature. h,i, Liver; j,k, adipose tissue;
l,m, pancreas. Results are from one independent experiment (a–m). DC, dendritic cell; NK, natural killer; pDC, plasmacytoid dendritic cell; ASPC, adipose progenitor and stem cells.
It was also demonstrated how well uPAR CAR T-cells eliminate uPAR-positive senescent cells. Therefore, tissue pathology or alterations in the renal and hepatic parameters in elderly mice had no bearing on the effectiveness of uPAR CAR T-cells.
In mice that were fed a high-fat diet and were naturally ageing, the activity of uPAR CAR T-cells was found to be associated with enhanced glucose homeostasis and metabolic fitness. Significantly, once uPAR CAR T-cells were administered at the prescribed doses, no toxicity was seen.
Yet another startling finding was the ability of uPAR CAR T-cells to prevent age- and diet-related metabolic decline and decrease. Furthermore, uPAR CAR T-cells show enduring impacts on small-molecule-based senolytic techniques. These cells were able to reduce the age- or HFD-induced metabolic syndrome in mice treated as young patients or those given an HFD after only one treatment.
Senescent pancreatic beta cells are eliminated in senolytic research, which has implications for glucose tolerance. Senescence of immune cells, however, might also have been involved.
It has also been proposed that eliminating senescent-featured macrophages can mitigate tissue deterioration in mice. The findings of the study, which show that some uPAR-expressing macrophages coexpress transcriptional patterns linked to senescence and senescence-associated β-galactosidase (SA-β-gal), are in line with this.
In conclusion
Small drugs’ mechanisms of action are frequently poorly understood; on the other hand, because senolytic CAR T-cells express particular surface antigens, their underlying mechanisms are well characterised. Compared to small-molecule or vaccine techniques, this strategy has many advantages because cellular therapies control persistence through different CAR designs and have safety switches built in.
These antigens may be the focus of cellular therapy in the future to treat various phenotypes. The enduring impact and endurance of uPAR-targeted CAR T-cells following a solitary infusion portend favourably for the senolytic CAR T-cell method of managing persistent diseases.