Strong recruiting techniques and Strategies for Clinical Trials begin with the patient as the center of attention and use a range of outreach approaches that are aimed to educate and engage prospective participants. Before beginning outreach, it is a good idea to establish a systematic strategy for the recruitment of clinical trial participants.
This is a smart method to guarantee that the process is organized, and productive, and places the utmost importance on the patient experience. When it comes to recruiting for clinical research, speed is key in addition to putting the patient’s needs first.
It is estimated that a delay in the development of a drug can cost sponsors approximately $37,000 per day in operational costs and $600,000 to $8M per day in opportunity costs. It can be extremely important for patients who are dealing with serious conditions to gain access to a more effective treatment option as soon as possible.
The Strategies for Clinical Trials recruiting that are shown here are intended to assist clinical trial sponsors in meeting or exceeding their recruitment deadlines and connecting patients with research opportunities that are a suitable match for them.
Clinical trial market
The market for clinical trials is quite large and is expanding very quickly. In the year 2000, a total of 2,119 active studies were registered on clinicaltrials.gov; as of right now, the website has a total of 280,000 current clinical research studies. This suggests that the value of the market is increasing, and it is anticipated that it will reach $65.2 billion by the year 2025.

According to a study produced by the United States Department of Health and Human Services, the overall cost involved with Strategies for Clinical Trials throughout all phases might vary anywhere from $44 million to $115.3 million. This estimate is based on the therapeutic area in which the medicine would be used. … only around one out of every ten medications that are tested in clinical trials really makes it to market.
Statistics of patient recruitment failures
The number of people participating in clinical trials is growing, but there are still significant barriers to participant recruitment. Here are some intriguing data.
• The National Institutes of Health (NIH) reports that more than 80 percent of clinical trials conducted in the United States do not reach the patient recruiting deadlines they have set for themselves. Because of these delays, expenses will grow, resources may be depleted, and it will take longer to reach the market.
• Up to 86 percent of Strategies for Clinical Trials studies do not meet their recruiting objectives within the allotted amount of time.
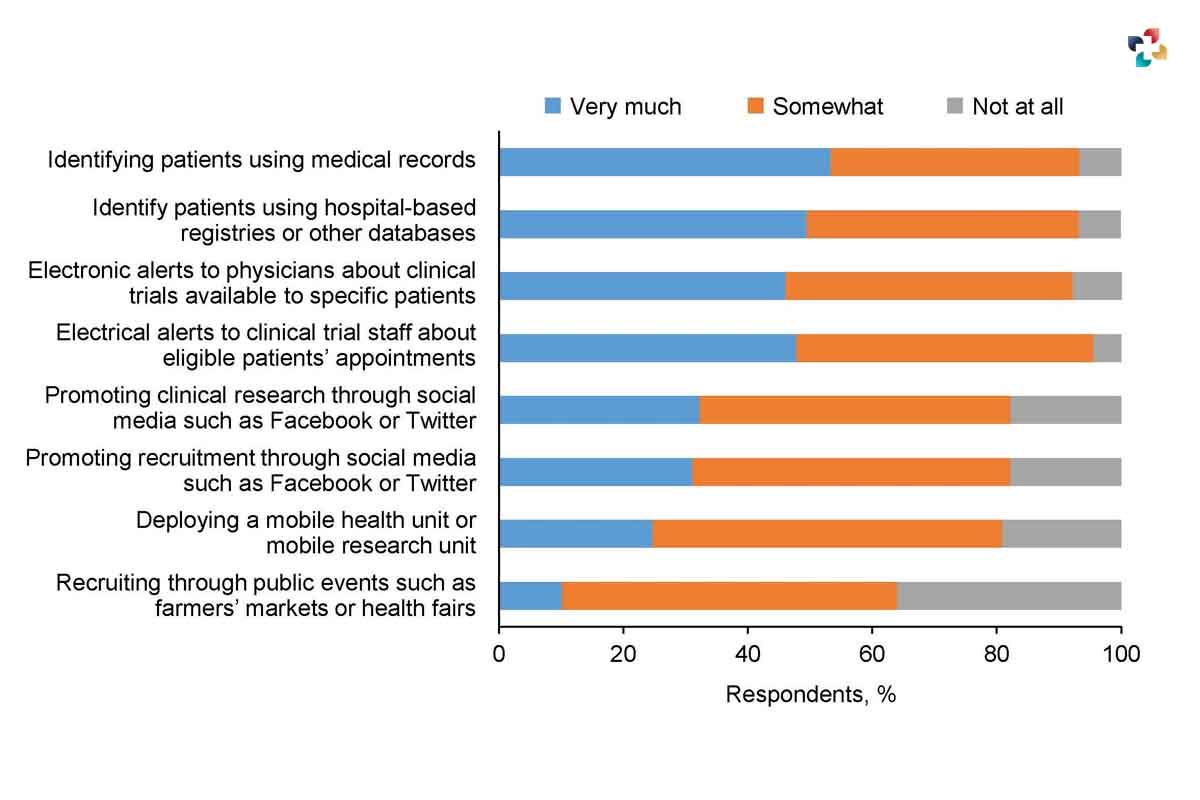
• According to a study that looked at registered studies, 19% of those trials had to be scrapped or ended prematurely because the researchers were unable to recruit enough people to participate.
• According to the FDA, only six percent of clinical studies are finished on schedule, and eighty percent of all trials are postponed by at least one month due to insufficient participant recruitment.
Patient recruitment for clinical trials
If a clinical study is unable to enroll a significant number of participants, then it will be impossible to make reliable conclusions about the results of the trial, and even promising treatments may seem to have failed. The recruitment of patients for clinical studies is contingent on a number of different criteria.
• Clinical investigators’ capabilities, such as investigator understanding of trial requirements, number of patient visits, inexperience in the recruitment of patients for trials, etc.
• Clinical trial site reach to the number of patients, travel distances from home, patient pool available for recruitment
• Clinical trial design that can engulf more patients (for example, unnecessary restrictive inclusion and exclusion criteria may reduce patient recruitment scope)
• Clinical trial design that can engulf more patients (for example, unnecessary restrictive inclusion and exclusion criteria may reduce

Here Are 10 Strategies for Clinical Trials;
1. Create recruiting materials
Create recruiting materials that particularly target the audience you are attempting to acquire, such as brochures or movies in their native language. This may be accomplished by developing recruitment materials that precisely target the demographic you are seeking to recruit.
2. Social media
Reaching out to prospective participants and sharing information about the research by using social media platforms such as Facebook, Twitter, and Instagram is one of the best Strategies for Clinical Trials.
3. Community groups
Establish collaborative relationships with community groups Form collaborative relationships with organizations that serve the demographic you are attempting to recruit, such as churches or community centers, to assist in disseminating information on the research project.
4. Conventional forms of media
Make use of the conventional forms of media in Strategies for Clinical Trials by publicizing the research project through radio, television, and print publications such as newspapers.
5. Communicate with medical professionals
Communicating with medical professionals and asking them to suggest people who could be eligible for the research is a great choice in Strategies for Clinical Trials.
6. Make use of patient advocacy organizations
Form partnerships with patient advocacy groups in order to assist in disseminating information about the research and provide details to the organizations’ respective members.
7. Make use of internet advertising
Publicize the study on websites and social media platforms that are widely used by the group of people that you are attempting to enlist for the research.
8. Develop a website dedicated to the study
Create a website that is solely devoted to the study and that includes information, a frequently asked questions section, and a mechanism for prospective participants to get in touch with the research team.
9. Use email and text message campaigns
Inform prospective participants about the research and how to participate in it by sending them information through email or text message campaigns.
10. Provide possible participants with incentives
Provide potential participants with incentives, such as gift cards or transportation reimbursement, in order to motivate them to take part in the research.
Also Read: 5 things to know about Clinical Trials







