Life is made up of many complex chemical reactions happening in our bodies. One important process is called oxidative phosphorylation, which helps produce most of the energy our cells need in a form called ATP (adenosine triphosphate). This process occurs in the mitochondria, which are known as the “powerhouses of the cell.” It involves two main steps: transporting electrons and a process called chemiosmosis. Together, these steps help create the energy that keeps our cells functioning properly.
Understanding the Fundamentals
Before delving into the intricacies of oxidative phosphorylation, it’s crucial to grasp some fundamental concepts:
- Cellular Respiration: This overarching process involves the breakdown of organic molecules, primarily glucose, to extract energy. It encompasses three key stages: glycolysis, the citric acid cycle (Krebs cycle), and oxidative phosphorylation.
- Mitochondria: These double-membrane-bound organelles are the primary sites of oxidative phosphorylation. The inner membrane folds inward, creating cristae that significantly increase the surface area for crucial enzyme complexes.
- Electron Transport Chain (ETC): A series of protein complexes embedded within the inner mitochondrial membrane, the ETC acts as a conveyor belt for electrons, passing them from one complex to another.
- Chemiosmosis: This process harnesses the energy generated by electron transport to drive the synthesis of ATP.
The Dance of Electrons: Electron Transport Chain
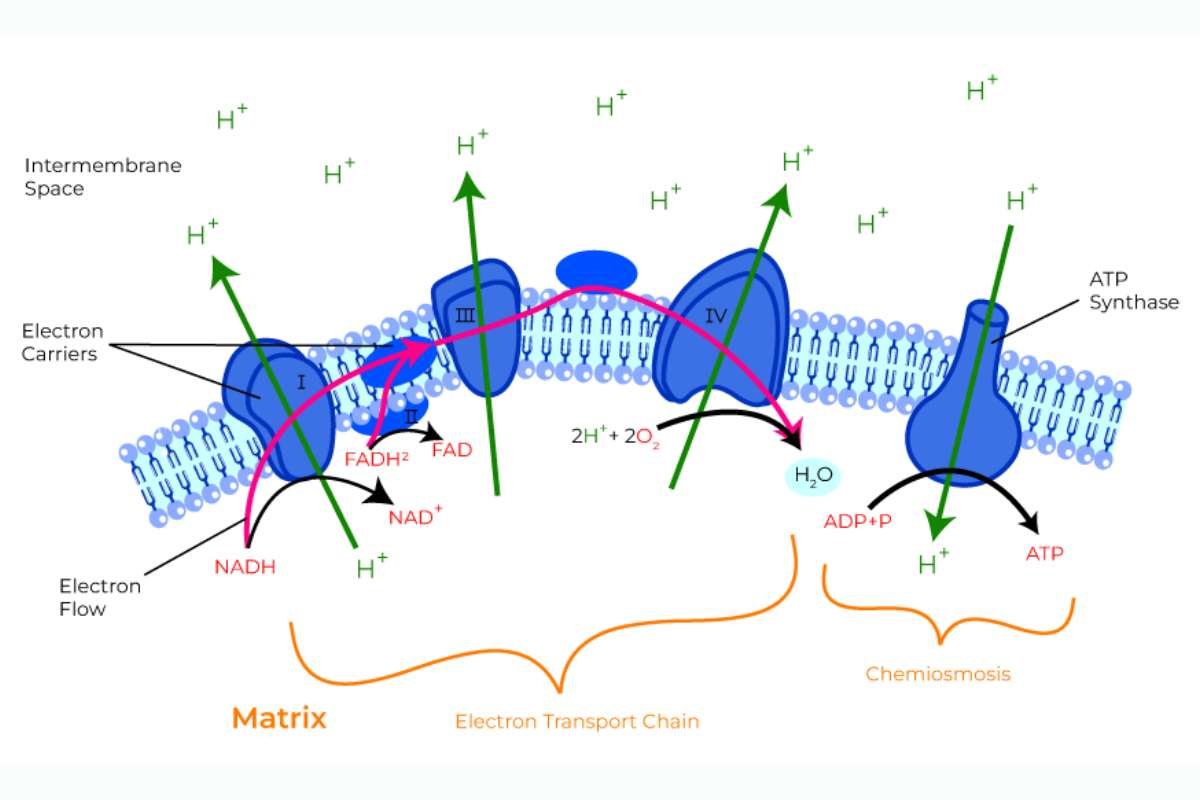
Oxidative phosphorylation starts with a process called the electron transport chain (ETC). This process uses high-energy electrons that come from breaking down glucose during earlier steps called glycolysis and the citric acid cycle. Special molecules known as electron carriers, like NADH and FADH2, transport these electrons to the ETC. When the electrons enter the first protein complex in the chain, they trigger a series of reactions. As the electrons move through the ETC, they lose energy at each step. This lost energy is used to push protons (H+) from inside the mitochondria to the space between the inner and outer membranes.
This movement creates a difference in proton concentration, with more protons outside than inside. This difference acts like stored energy, similar to how water behind a dam holds potential energy. This setup is crucial for producing ATP, the energy currency of our cells.
The ATP Synthase: Harnessing the Proton Gradient
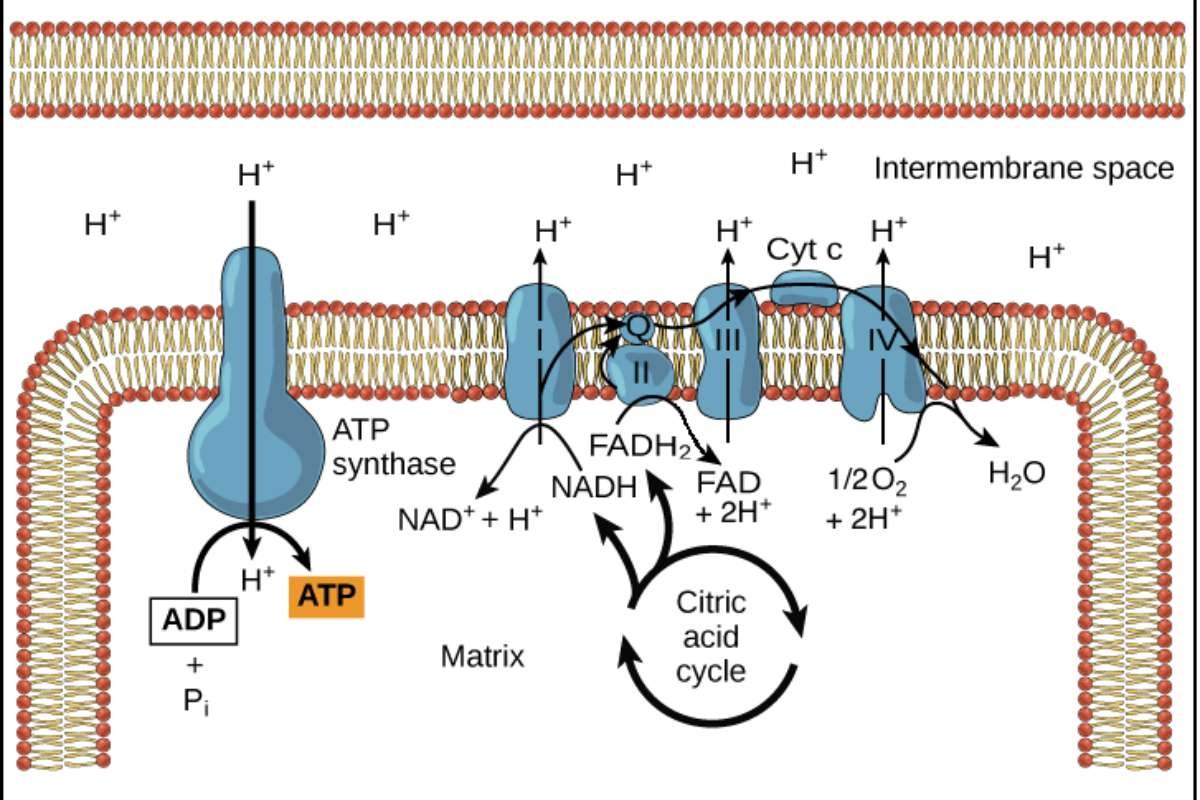
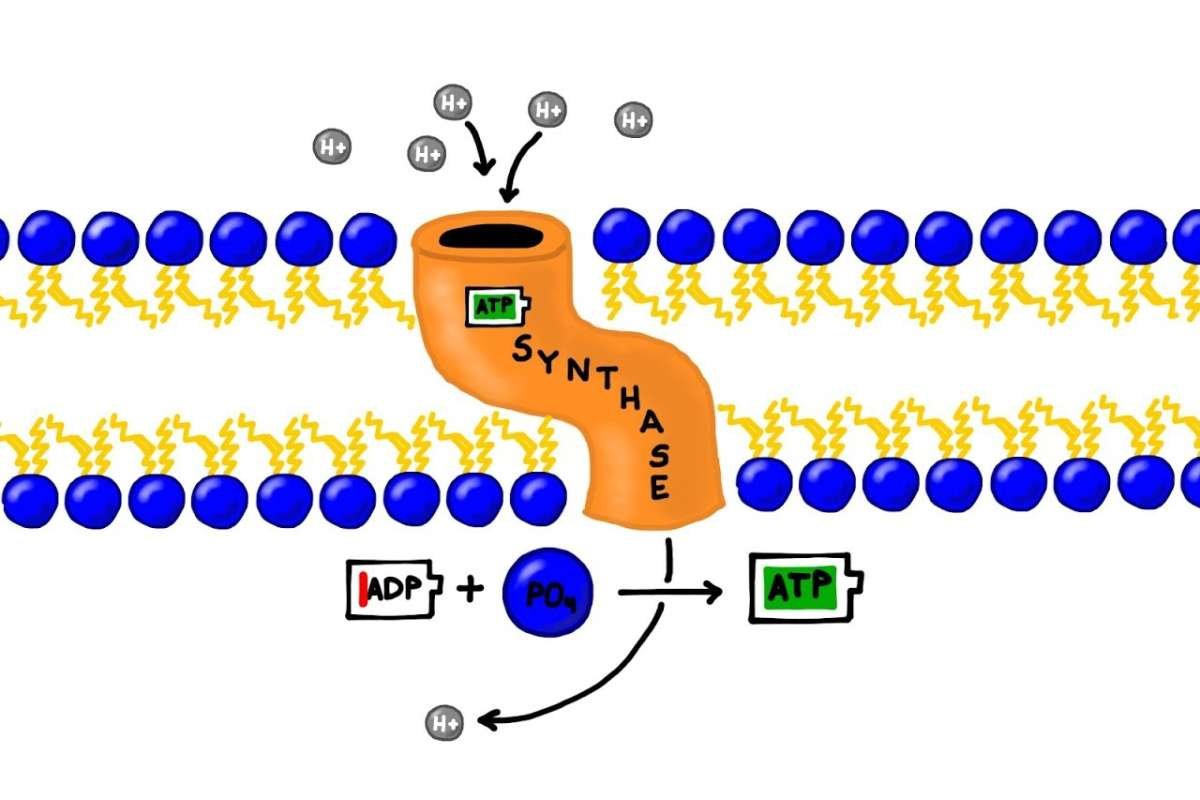
The proton gradient created by the electron transport chain helps power the last part of oxidative phosphorylation, called chemiosmosis. An important enzyme called ATP synthase is found in the inner mitochondrial membrane. It works like a tiny motor that uses the proton gradient to make ATP.
As protons move back into the mitochondrial matrix through a channel in ATP synthase, they create a flow that makes the enzyme spin. This spinning action helps combine ADP and inorganic phosphate (Pi) to produce ATP.
This process is very efficient and works like a waterwheel turning to grind grain, resulting in a large amount of ATP being made for the cell’s energy needs.
The Significance of Oxidative Phosphorylation
Oxidative phosphorylation is of paramount importance for cellular life. It provides the vast majority of the ATP required to fuel various cellular activities, including:
- Muscle contraction: ATP is essential for the sliding filament mechanism that powers muscle movement.
- Cell signaling: Many signaling pathways rely on ATP to drive protein phosphorylation and other cellular responses.
- Active transport: ATP is utilized to pump molecules across cell membranes against their concentration gradients.
- DNA replication and repair: These vital processes require significant energy input, which is provided by ATP.
- Biosynthesis: ATP is used to synthesize various biomolecules, including proteins, lipids, and carbohydrates.
Inhibitors of Oxidative Phosphorylation
Several compounds can interfere with oxidative phosphorylation, disrupting cellular energy production and leading to serious consequences. These inhibitors include:
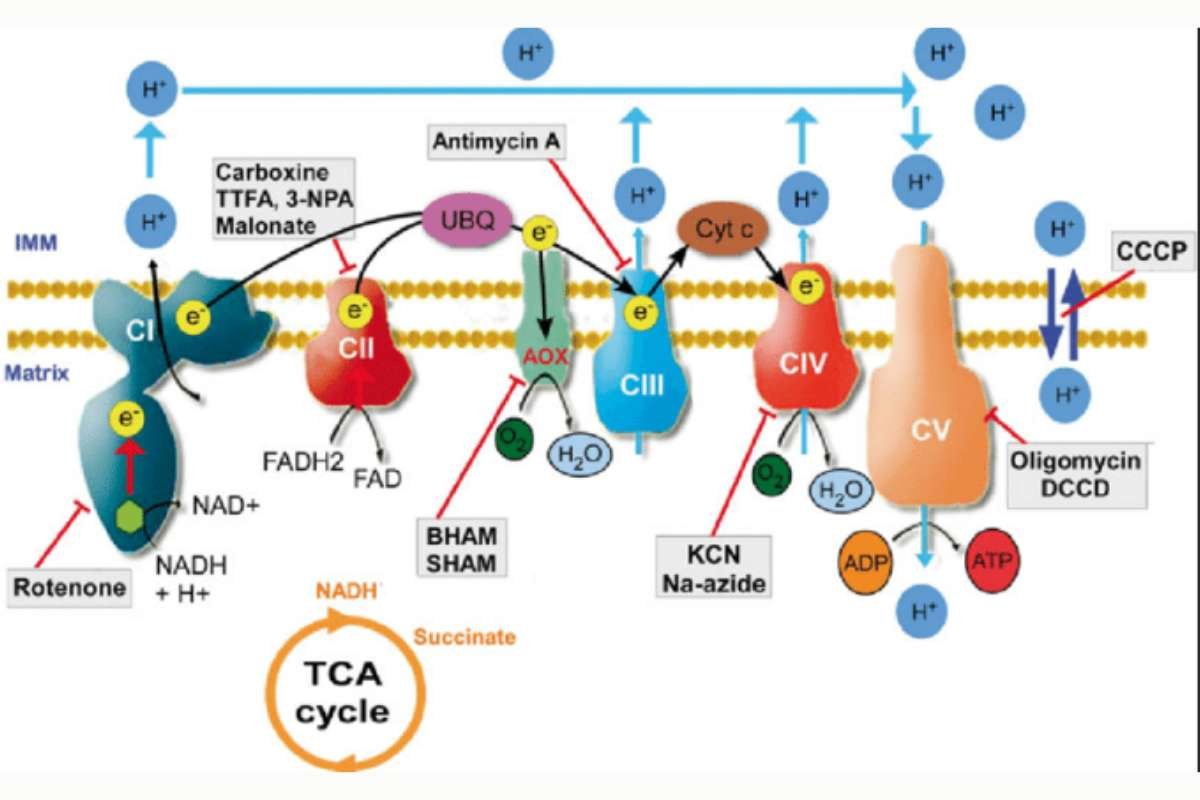
- Cyanide: This potent poison binds to cytochrome c oxidase, the final complex in the ETC, blocking electron flow and halting ATP synthesis.
- Carbon monoxide: This gas also binds to cytochrome c oxidase, inhibiting its function and leading to cellular energy depletion.
- Oligomycin: This antibiotic inhibits ATP synthase, preventing the utilization of the proton gradient for ATP synthesis.
- Rotenone: This natural insecticide inhibits complex I of the ETC, blocking electron flow and disrupting energy production.
Dysfunction of Oxidative Phosphorylation and Human Disease
Problems with oxidative phosphorylation can seriously affect human health. Mitochondrial diseases are a group of genetic disorders that often happen because of issues with the proteins in the mitochondria that are involved in the electron transport chain or ATP production. These diseases can cause various symptoms, such as muscle weakness, problems with the nervous system, and heart issues.
Additionally, as we age, our mitochondria gradually work less effectively, which leads to lower ATP production and higher oxidative stress. This decline in mitochondrial function may contribute to age-related diseases, including conditions that affect the brain, like neurodegenerative disorders, and heart diseases.
Conclusion
Oxidative phosphorylation is a great example of how well biological systems work. This complex process, which involves the teamwork of the electron transport chain and ATP synthase, is essential for keeping us alive. By knowing how oxidative phosphorylation works, scientists can better understand how cells produce energy. This knowledge can also help them find ways to treat diseases that are caused by problems in the mitochondria.