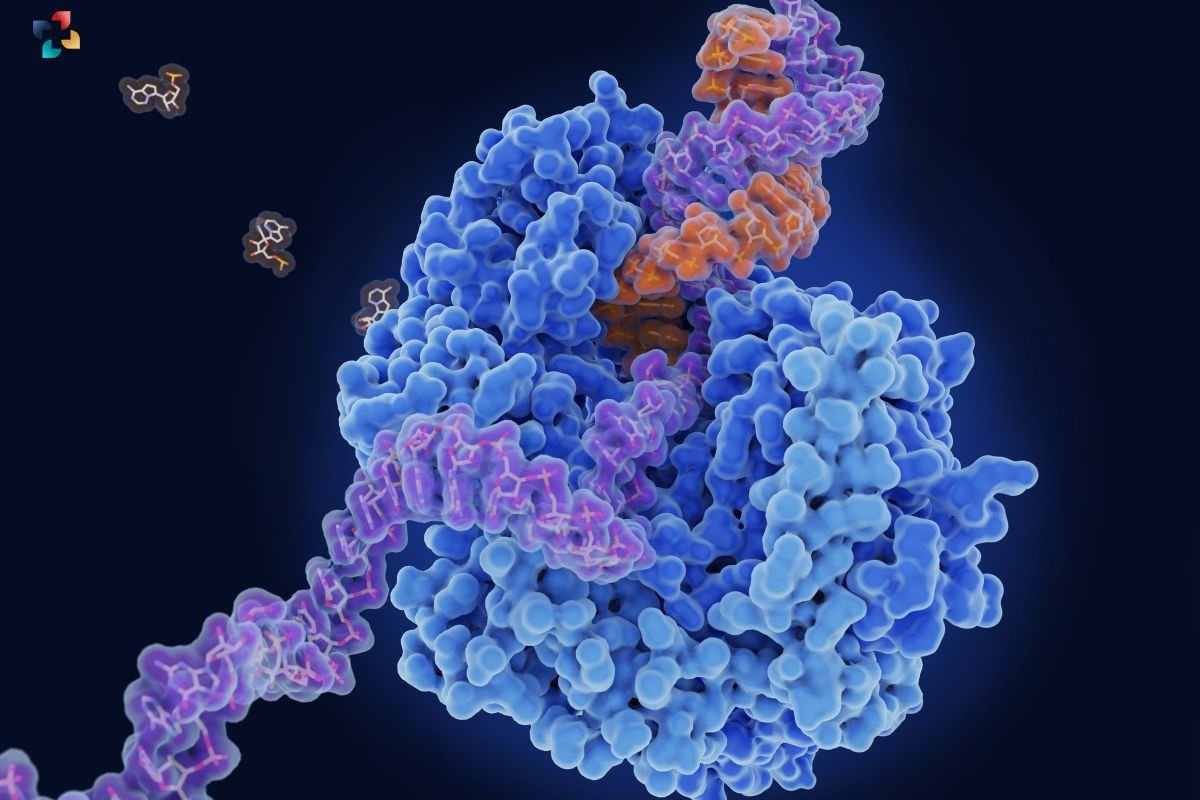
In a groundbreaking study published in Nature Structural & Molecular Biology, researchers have unveiled the intricate process by which RNA polymerase (RNAP) initiates transcription by opening the transcription bubble on DNA. This pivotal process marks the beginning of gene expression in all living cells, where RNAP binds to DNA and prompts the unwinding of the double helix to expose a single DNA strand for RNA synthesis.
For years, scientists have grappled with understanding how RNAP accomplishes this rapid feat. The process unfolds within milliseconds, making it challenging to capture with existing technologies. However, leveraging innovative methods, researchers managed to capture real-time snapshots of Escherichia coli RNAP as it opened the transcription bubble, shedding unprecedented light on the molecular mechanisms underlying transcription initiation.
Unraveling the Molecular Dance
The study, spearheaded by Professor Seth Darst and his team at Rockefeller University, utilized state-of-the-art cryo-electron microscopy and a rapid sample preparation system developed by collaborators at the New York Structural Biology Center. This technology allowed them to freeze-frame RNAP-DNA interactions mere milliseconds after their initial contact. Through meticulous analysis, the researchers identified four distinct intermediate complexes, each revealing crucial steps in how RNA Polymerase grips the DNA strands and stabilizes the transcription bubble formation.
One of the key findings was the dynamic interaction between RNA Polymerase and the DNA strands as they separate. The enzyme gradually locks onto one DNA strand, preventing the double helix from reannealing. Concurrently, structural changes in RNAP facilitate the formation of essential protein-DNA connections, culminating in the stable establishment of the transcription bubble. This phase is pivotal, as it dictates the speed at which RNAP proceeds to RNA synthesis, resolving longstanding debates about the rate-limiting steps in transcription initiation.
Implications for Future Research
Looking ahead, the study proposes that a critical rate-limiting step in transcription initiation involves positioning the DNA template strand precisely within RNAP’s active site. This step requires overcoming significant energy barriers and orchestrating complex molecular rearrangements. Future research aims to validate this hypothesis and delve deeper into subsequent stages of the transcription cycle.
The implications of this research extend beyond fundamental biology, offering a new paradigm for studying dynamic molecular interactions in real time. By elucidating how RNA Polymerase navigates the early stages of transcription, scientists can better understand essential cellular processes and potentially uncover new targets for therapeutic interventions.
“This study marks a significant leap forward in our understanding of transcription initiation,” remarks Andreas Mueller, a coauthor of the study. “Our next steps involve exploring additional complexes and later stages in the transcription process to comprehensively map out how gene expression is regulated.”
In conclusion, the study not only resolves longstanding mysteries about the initiation of transcription but also underscores the transformative potential of real-time imaging technologies in biological research. As Professor Darst aptly summarizes, “Understanding the intricacies of transcription initiation is crucial for unraveling the complexities of life itself.” This research paves the way for future discoveries that promise to deepen our understanding of cellular function and disease mechanisms.