Source – The Week
Addressing the Challenges of Liver Disease and Transplantation
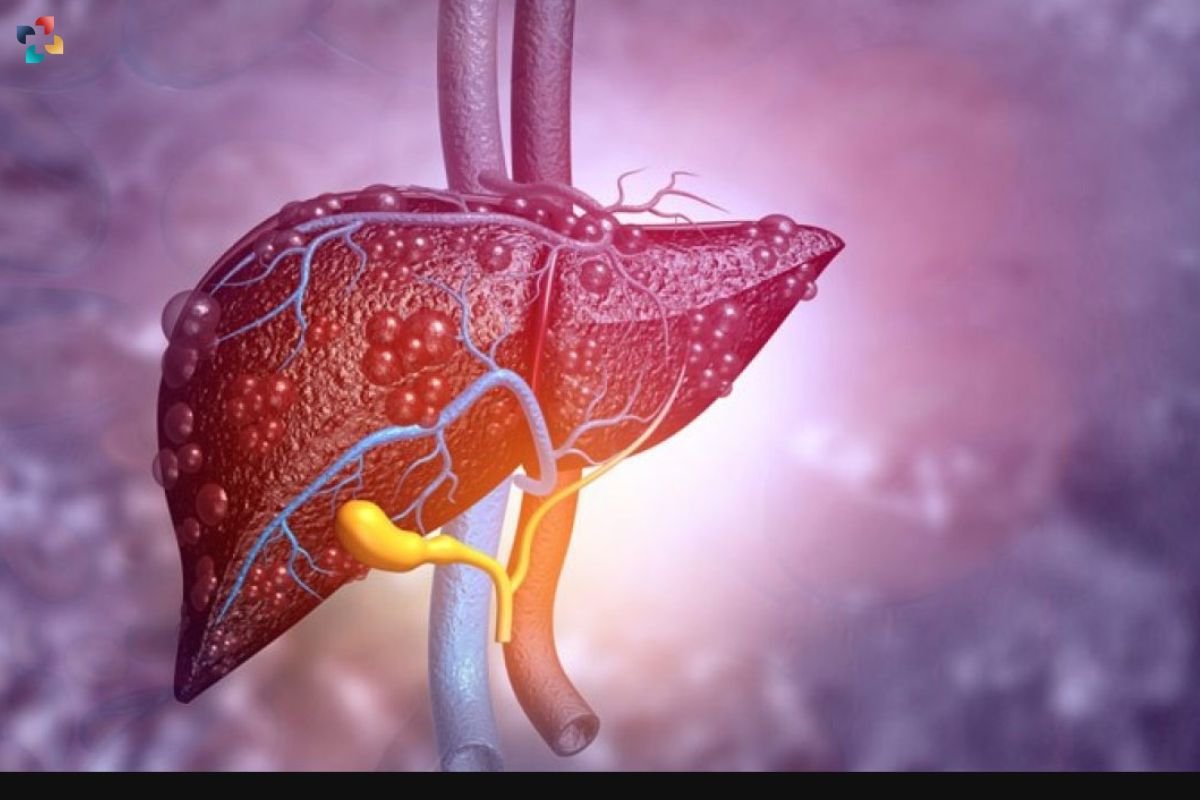
Liver disease remains a significant global health concern, contributing to 1 in every 25 deaths worldwide. While liver transplantation can be life-saving for those with end-stage liver disease, the procedure faces challenges such as donor shortage, surgical complexity, and the need for lifelong immunosuppressive medication. Alternative approaches, such as injecting dissociated human liver cells, have been explored, but donor scarcity persists as a limiting factor.
Innovative Solution: Cross-Species Liver Cell Growth
To overcome the barriers of donor shortage and enhance the availability of transplantable liver cells, researchers from the Chinese Academy of Sciences in Beijing embarked on an innovative approach. They utilized interspecies blastocyst complementation, injecting mouse embryonic stem cells into early rat embryos. This resulted in the birth of mouse-rat chimeras, containing mouse cells, including liver cells, within their bodies. Notably, the percentage of live mouse liver cells in the chimeric livers reached up to 20.6%.
Promising Functional Results and Future Directions
Encouragingly, the mouse liver cells grown in the mouse-rat chimeras exhibited normal appearance and mature function in laboratory tests. Moreover, when transplanted into mice with liver damage, these cells demonstrated a therapeutic effect comparable to normal mouse liver cells, effectively relieving chronic liver fibrosis. Published in Stem Cell Reports, these findings demonstrate the proof of principle that functional liver cells can be grown in a different species, offering a potential solution to the transplant shortage.
Future research will focus on refining techniques to grow human liver cells in large animals like pigs and assessing the functionality of these cells. The success of this innovative approach holds promise for addressing the critical shortage of transplantable liver cells and improving outcomes for patients with liver disease worldwide.